Abstract
Summary: We describe the use of a CT-based method of cerebral perfusion imaging, dynamic CT perfusion imaging, for the pre- and postprocedural assessment of cerebral blood flow in a patient with symptomatic middle cerebral artery occlusive disease who underwent balloon angioplasty with stent placement in the affected artery. Dynamic CT perfusion imaging represents a widely available and minimally invasive alternative to other available methods of cerebral perfusion imaging.
For patients with symptomatic cerebrovascular occlusive disease, perfusion imaging is often performed to select those patients who are likely to benefit from interventions designed to increase blood flow (1–4). However, many methods of perfusion imaging (such as positron emission tomography [PET] and xenon-enhanced CT) are not widely available outside large centers. Recently, a new method of cerebral perfusion imaging, dynamic CT perfusion imaging, was developed that uses equipment available in most radiology departments (5, 6). We report use of dynamic CT perfusion imaging with a pharmacologic vasodilative challenge for pre- and postprocedural evaluation of a patient with middle cerebral artery (MCA) occlusive disease. To our knowledge, the methods and findings we report have not been previously described in the literature.
Case Report
A 53-year-old man with history of stroke in the territory of the left MCA and recurrent episodes consisting of dizziness and right-sided numbness presented for an evaluation of his symptoms. Cerebral digital subtraction angiography revealed nearly complete obstruction of the proximal left MCA (Fig 1A).
Fig 1.
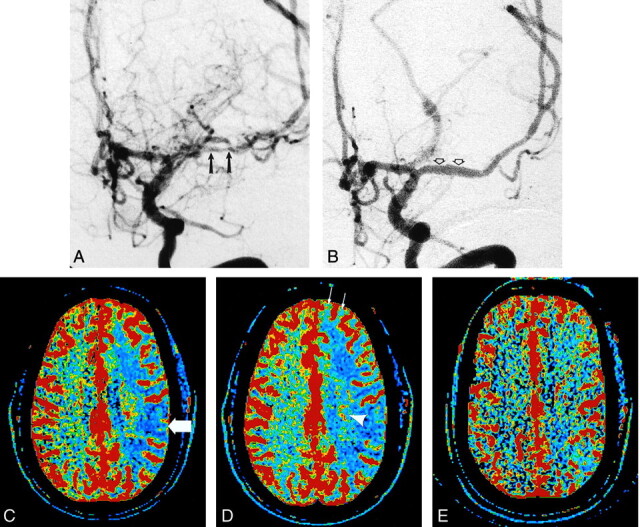
Images in a 53-year-old man with episodes of right-sided numbness.
A, Digital subtraction angiogram in the left anterior oblique projection following injection of the left internal carotid artery shows severe narrowing and irregularity of the left MCA (arrows).
B, Digital subtraction angiogram following angioplasty and stent placement in the left MCA shows a nearly normal caliber and appearance to the proximal left MCA (arrows).
C, Preprocedural CBF map before acetazolamide infusion shows decreased blood flow in the left hemisphere represented as extensive areas displayed as blue (arrow).
D, Preprocedural CBF map obtained 20 minutes after infusion of acetazolamide, with use of the same section location and display parameters as those in C. Despite appropriate response on the right, no increase in CBF is apparent in the left hemisphere. Instead, additional decrease in CBF can be seen in areas such as the basal ganglia (arrowhead) and frontal lobe (arrows). By using ROI analysis, an inappropriate (ie, paradoxical) decrease in mean cortical CBF values of 23% was measured on the left and was interpreted as evidence of severe hemodynamic impairment.
E, Postprocedural CBF map before infusion of acetazolamide shows greatly improved blood flow in the left hemisphere compared with that seen in C and D. Appropriate increases in mean cortical CBF values were measured in both hemispheres after repeat acetazolamide challenge (not shown).
Dynamic CT perfusion imaging was performed in the transverse plane with use of a multi–detector row CT scanner (Lightspeed; GE Medical Systems, Milwaukee, WI) to assess the hemodynamic status of the MCA territory in anticipation of MCA angioplasty. Two noncontiguous locations were selected for study: a lower location at the level of the basal ganglia and an upper location at the level of the lateral ventricles. At each location, a 2-cm-thick slab oriented in the transverse plane was defined and divided into four 5-mm-thick contiguous transverse sections, yielding a total of eight 5-mm sections, which were studied once before intravenous infusion of 1000 mg of acetazolamide (Diamox; Wyeth, Marietta, PA) and again 20 minutes after the infusion of acetazolamide. Because study of the upper and the lower locations had to be done separately, a total of four acquisitions of data were performed. For each of the four acquisitions, 40 mL of nonionic iodinated contrast material (Isovue 370; Bracco Diagnostics,) was infused at 4 mL/s over 10 seconds through an 18-gauge antecubital catheter. Scanning began 5 seconds after the start of the infusion of contrast material and continued for 45 seconds. A continuous (cine) mode of acquisition (1 second per revolution) was used at each location with no table motion during data acquisition. X-ray generation was performed with use of 80 kVp and 200 mA.
Data were retrospectively reconstructed at 0.5-second intervals and transferred to an imaging workstation (Advantage Windows, GE Medical Systems) operating commercial software designed for analysis of CT perfusion imaging data (CT Perfusion, GE Medical Systems). The deconvolution-based algorithm in this software was chosen because it is thought to be more accurate than other available methods of analysis (5).
For each of the eight sections studied, an experienced neuroradiologist (J.D.E.) created hand-drawn regions of interest (ROIs) on a reference CT image from the cine data set over the cortical gray matter of the expected territory of the MCAs bilaterally, taking care to exclude large cortical blood vessels. Maps of the cerebral blood flow (CBF) were then created with the ROIs in place. The area and mean CBF value contained within each ROI were recorded. To compare the two hemispheres, mean CBF values on the right and on the left for all eight sections were computed by weighting the perfusion parameter values measured on each section by the areas of the ROIs that contained them.
On the preprocedural CT perfusion images, the mean value of cortical CBF measured in the right (normal) hemisphere was 66.4 mL/100 g/min before acetazolamide and 74.7 mL/100 g/min after acetazolamide. The mean value of cortical CBF in the left (affected) hemisphere was 40.7 mL/100 g/min before acetazolamide and 31.4 mL/100 g/min after acetazolamide. Maps of CBF before and after acetazolamide are shown in Figure 1C and D.
Because the results of the initial CT perfusion study were taken as evidence that severe hemodynamic impairment was present in the left MCA territory, the patient was provided with options for treatment, including angioplasty and stent placement in the left MCA. Twenty days later, the patient underwent percutaneous transluminal angioplasty of the left MCA with use of a 1.5 × 10-mm CrossSail balloon catheter (Guidant, Temecula, CA) and placement of a 2.5 × 9-mm AVE S660 wire mesh stent (Medtronic AVE, Santa Rosa, CA) across the region of prior stenosis, without complication (Fig 1B). One day after intervention, repeat CT perfusion imaging was performed by using the same protocol as the that of the preprocedural perfusion study.
The repeat CT perfusion study after intervention showed that cortical CBF in the right hemisphere was 48.9 mL/100 g/min before acetazolamide and was 54.4 mL/100 g/min after acetazolamide. The mean value of cortical CBF in the left hemisphere was 47.3 mL/100 g/min before acetazolamide and 51.3 mL/100 g/min after acetazolamide. The postprocedural CBF map is shown in Fig 1E. The patient had no recurrence of symptoms during the 6 months after the procedure.
Discussion
Occlusive disease of cerebral blood vessels may result in symptoms such as stroke and transient ischemic attack (1–4). Prevention of further symptoms is the goal of therapy, although the optimal therapy for an individual patient (eg, anticoagulant therapy vs intervention designed to increase CBF) is frequently not known. Thus, selection of patients who are most likely to benefit from therapeutic interventions such as angioplasty and stent placement is an important goal.
Most of the methods available for quantitative assessment of cerebral hemodynamic function, such as PET and xenon-enhanced CT, are available only at large centers that have specialized equipment. MR perfusion imaging can also be used to assess cerebral hemodyamics, but it requires specialized hardware (rapid imaging gradients and MR-compatible contrast material injectors) that may not routinely be available. A quantitative perfusion imaging method that uses equipment presently existing in most radiology departments would, therefore, be valuable.
Slip-ring design CT scanners (the type of scanners used to perform dynamic CT perfusion imaging studies) are available in most radiology departments. Dynamic CT perfusion studies are also rapid, typically taking fewer than 15 minutes to perform (including the acquisition of setup and scout views) and fewer than 10 minutes to analyze. Another advantage of dynamic CT perfusion imaging compared with other available methods of perfusion imaging is that it can be performed rapidly in conjunction with nonenhanced CT, which is typically performed in the emergency evaluation of patients with acute stroke.
Published studies (5, 6) have described the use of dynamic CT perfusion imaging in the assessment of patients with acute stroke and those with vasospasm related to subarachnoid hemorrhage. However, we are unaware of published reports that describe the use of this method for preprocedural assessment of patients with chronic cerebrovascular insufficiency. We are also unaware of published reports of the use of dynamic CT perfusion imaging in association with a pharacologic challenge (acetazolamide challenge).
Large cortical blood vessels were excluded from the ROIs drawn in this study because it was assumed that inclusion of large blood vessels would decrease accuracy. In our experience, CBF measurements performed with use of ROIs that include large blood vessels are more highly variable than those that do not include large blood vessels.
The infusion of acetazolamide, a potent inhibitor of the enzyme carbonic anhydrase, has been used for more than a decade for the evaluation of cerebral hemodynamics (1, 7). Although its precise mechanism of action is not known with certainty, acetazolamide infusion increases CBF by causing vasodilation of cerebral arterioles (8). However, in the setting of decreased perfusion pressure (eg, due to vascular occlusion), cerebral arterioles may already be dilated, owing to autoregulation of blood flow. Thus, further dilation of cerebral arterioles in response to acetazolamide may not occur. Possible abnormal responses in this situation may include a less than expected increase in blood flow or a paradoxical decrease in blood flow (likely due to shunting of blood away from the underperfused region [ie, steal phenomenon]) (2).
Adverse responses to infusion of acetazolamide are rare. The most common symptom associated with the infusion of acetazolamide is transient perioral tingling or numbness. A case of transient neurologic deficit associated with acetazolamide infusion has been reported (9).
For the patient described in the present study, the preprocedural CT perfusion study provided evidence of hemodynamic impairment in the territory of the left MCA. The CT study performed before acetazolamide infusion showed decreased relative CBF on the affected side, with a further decrease in relative CBF seen on the CT study after acetazolamide. On the basis of the preprocedural study, it was concluded that vasodilative reserve was substantially impaired (placing the patient at increased risk of stroke) and that intervention was warranted.
After angioplasty and stent placement in the left MCA, the CT perfusion study obtained before acetazolamide infusion showed improvement in CBF in the left hemisphere, and the CT study obtained after acetazolamide showed normal hemodynamic response. Both findings were interpreted to be desired responses to therapy.
Conclusion
We describe the use of dynamic CT perfusion imaging, in combination with a pharmacologic vasodilative challenge, for the assessment of cerebral hemodynamics before intervention to increase CBF. Dynamic CT perfusion imaging is already a highly promising technique for the emergency evaluation of patients with acute stroke. As this report indicates, this technique may also offer a convenient and widely available alternative to other perfusion imaging modalities for determining need for, and results of, therapy.
References
- 1.Vorstrup S, Brun B, Lassen NA. Evaluation of the cerebral vasodilatory capacity by the acetazolamide test before EC-IC bypass surgery in patients with occlusion of the internal carotid artery. Stroke 1996;17:1291–1298 [DOI] [PubMed] [Google Scholar]
- 2.Kuroda S, Kamiyama H, Abe H, Houkin K, Isobe M, Mitsumori K. Acetazolamide test in detecting reduced cerebral perfusion reserve and predicting long-term prognosis in patients with internal carotid artery occlusion. Neurosurgery 1993;32:912–919 [DOI] [PubMed] [Google Scholar]
- 3.Derdeyn CP, Cross DT, Moran CJ, Dacey RG. Reversal of focal misery perfusion after intracranial angioplasty: case report. Neurosurgery 2001;48:436–440 [DOI] [PubMed] [Google Scholar]
- 4.Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg 1993;79:483–489 [DOI] [PubMed] [Google Scholar]
- 5.Nabavi DG, LeBlanc LM, Baxter B. Monitoring cerebral perfusion after subarachnoid hemorrhage using CT. Neuroradiology 2001;43:7–16 [DOI] [PubMed] [Google Scholar]
- 6.Koenig M, Klotz E, Luka B, et al. Perfusion CT of the brain: diagnostic approach for early detection of ischemic stroke. Radiology 1998;209:85–93 [DOI] [PubMed] [Google Scholar]
- 7.Rogg J, Rutigliano M, Yonas H, Johnson DW, Pentheny S, Latchaw RE. The acetazolamide challenge: imaging techniques designed to evaluate cerebral blood flow reserve. AJNR Am J Neuroradiol 1989;19:803–810 [DOI] [PubMed] [Google Scholar]
- 8.Mchedlishvili G. Arterial behavior and blood circulation in the brain. New York, NY: Plenum Press;1986. :338
- 9.Komiyama M, Nishikawa M, Yasui T, Sakamoto H. Reversible pontine ischemia caused by acetazolamide challenge. AJNR Am J Neuroradiol 1997;18:1792–1784 [PMC free article] [PubMed] [Google Scholar]


