Abstract
BACKGROUND AND PURPOSE: Guglielmi detachable coils (GDCs) are effective in preventing rebleeding of ruptured aneurysms. To better understand the mechanism underlying this protective effect, we evaluated blood flow in aneurysms and their parent arteries before and after GDC coil placement.
METHODS: An 0.14-inch guidewire containing a combined pressure and thermistor sensor was inserted through a microcatheter into both the parent artery and the dome of surgically created canine aneurysms. Before and after GDC coil placement, intravascular pressures and thermodilution responses where recorded in the parent artery and aneurysmal dome during injections of room-temperature isotonic sodium chloride solution over 4 seconds (5 mL/s) and 2 seconds (20 mL/s) in the parent artery.
RESULTS: Before GDC coil placement, similar U-shaped thermodilution curves were present in the parent artery and in the dome of the aneurysm. GDC coil placement reduced intraaneurysmal flow by 61–99.6% (P < .05), prolonged aneurysmal filling and washout (dilution interval increased from 5.16 seconds before coil placement to 26.79 seconds after coil placement, P < 0.05), and caused a dissociation of pressure and flow (shift ε was 0.45 seconds before coil placement versus 0.56 seconds after coil placement, P < .05). Flow in the parent artery was not significantly affected by GDC coil placement in the aneurysm.
CONCLUSION: In this model, intraaneurysmal blood flow can be evaluated with thermodilution. GDC coil placement significantly reduces blood flow in aneurysms.
Aneurysms rupture when the stress on a portion of the wall exceeds the strength of the wall. The hemodynamic factors that induce wall stress include pressure, shear stress, and impulse, with pressure being the factor most often implicated in aneurysmal rupture. This proposition gains support from clinical observations of aneurysmal rupture in situations that are associated with transiently increased arterial pressure and heart rate, as well as from the pathologic observation that the site of most frequent rupture, the dome, is remote from the location of maximum shear stress and impulse (1–4).
Treatment of ruptured aneurysms with Guglielmi detachable coils (GDC) is effective in preventing aneurysmal rebleeding (5). Findings from both in vitro and in vivo animal studies have shown that coil placement in experimental aneurysms does not reduce mean aneurysmal pressure (6, 7). Recently, using an experimental canine aneurysm model that allows pressure measurement without disruption of the aneurysm wall, we confirmed these observations and demonstrated that, at both baseline and during periods of induced hypertension, aneurysmal pressure tends to increase slightly after coil placement. Moreover, the amplitude of the pressure inside the aneurysm is attenuated (8). It is uncertain, however, if these relatively moderate changes in pressure alone are responsible for the protective effect of GDCs against rebleeding.
The pattern of blood flow in a saccular aneurysm is determined by the geometric properties of the aneurysm, its parent artery, and adjacent branches (9, 10). In lateral (side-wall) aneurysms, inflow occurs during systole at the downstream extent of the ostium. Filling of the aneurysm proceeds in a cranial-to-caudal fashion along the aneurysm wall, with outflow occurring during diastole at the upstream extent of the ostium. A central vortex, separate from the flow around the margins of the aneurysm, fills and empties slowly. Flow in the parent artery adjacent to the aneurysm ostium is disturbed (9, 10). Because of this flow pattern, the levels of shear stress vary throughout an aneurysm; maximum levels occur at the neck (inflow zone), and minimal levels occur in the dome (11, 12). Because findings from studies of in vitro models have shown that even incomplete coil placement profoundly disturbs the aneurysmal flow pattern (13), we wanted to study this phenomenon further in our animal model by using a thermodilution technique.
Methods
Measurement of Aneurysm Flow
Under an institutional animal use protocol, lateral and bifurcation aneurysms were created in three mongrel dogs by using a technique originally described by German and Black (14) and later modified in our laboratory (15). At least 3 weeks passed between the time of aneurysm creation and treatment. Four side-wall aneurysms and one bifurcation aneurysm were studied.
Endotracheal halothane anesthesia was used in all instances. Vascular access was obtained by using a sterile technique and 6F sheaths placed by means of cutdown into both common femoral arteries. Through one of these, a 6F guiding catheter was positioned in the common carotid artery well proximal to each of the aneurysms. Fluoroscopy and digital subtraction angiography (DSA) were performed by using a portable C-arm unit. Arterial pressure and flow measurements were monitored and recorded throughout each experiment by using Run-Time LabView 5.1 software (National Instruments).
After placement of the guiding catheter into the parent artery of each aneurysm, a 0.014-inch guidewire-mounted pressure sensor (PressureWire Sensor; Radi Medical Systems, Uppsala, Sweden) was positioned in the parent artery so that the transducer was located 2–4 cm proximal to the aneurysm (Fig 1). Another 0.014-inch guidewire-mounted combined pressure-temperature sensor was calibrated to zero at room temperature and placed in the dome of the aneurysm (Fig 1). Blood flow was evaluated by using the thermodilution technique. With a commercially available angiographic injector (Angiomat 3000; Liebel-Flarsheim, Cincinnati, OH), 5 and 20 mL of room-temperature isotonic sodium chloride solution was injected into the parent artery of the aneurysm over 4 and 2 seconds, respectively.
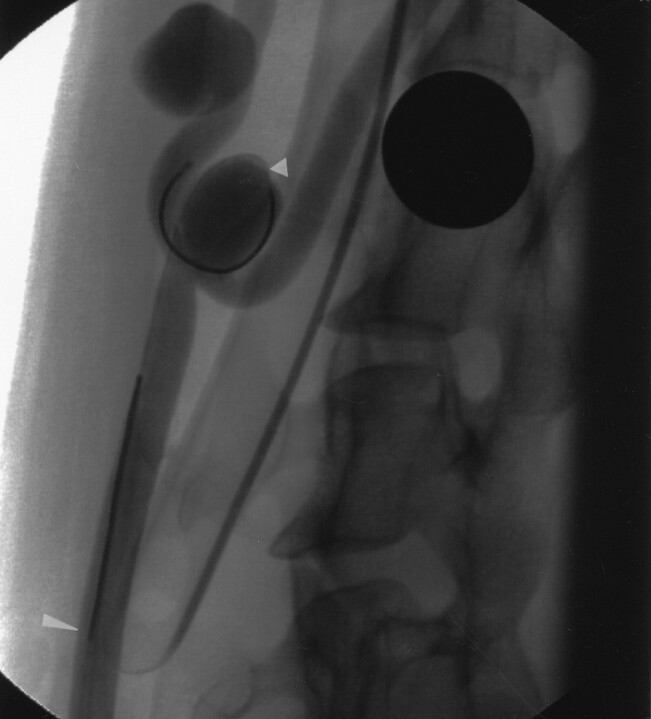
Fig 1.
DSA image shows the experimental aneurysm model used in this study. Guidewires that were used to measure the temperature and pressure is present in the bifurcation aneurysm (short arrowhead) and parent artery (long arrowhead).
The observed decrease in the temperature in the dome of the parent artery is proportional to the volume flow rate at the point of measurement (16, 17). To evaluate the flow in the parent artery, the position of the two measuring devices was reversed (ie, the pressure and temperature sensors were placed in the parent artery, and the pressure measurement sensor was placed in the aneurysmal dome). The guidewires were then repositioned so that the pressure-temperature sensor again was in the dome of the aneurysm. Exact positioning of the pressure sensor and maintenance of the specific placement was accomplished by identifying the radiopaque marker of the sensor with use of a DSA roadmap.
With the pressure-temperature wire in place, a Tracker Catheter (Target Therapeutics/Boston Scientific) was introduced into the aneurysm, and GDCs were positioned and detached by using standard clinical techniques. Intraaneurysmal flow was evaluated after insertion of the first coil and after placement of the final coil. All aneurysms were treated by using clinical criteria so that packing was continued until no more coils could be introduced into the aneurysm or until a risk of compromising the parent artery was present. Finally, the guidewire with the pressure sensor in the parent artery was removed, and a guidewire with a combined pressure-temperature sensor was inserted. Room-temperature injections of sodium chloride solution were repeated in a manner identical to that before the start of coil placement. After completion of these measurements, all animals were euthanized with intravenous administrations of Beuthanasia D 1 mL per 5 lbs.
Data Analysis
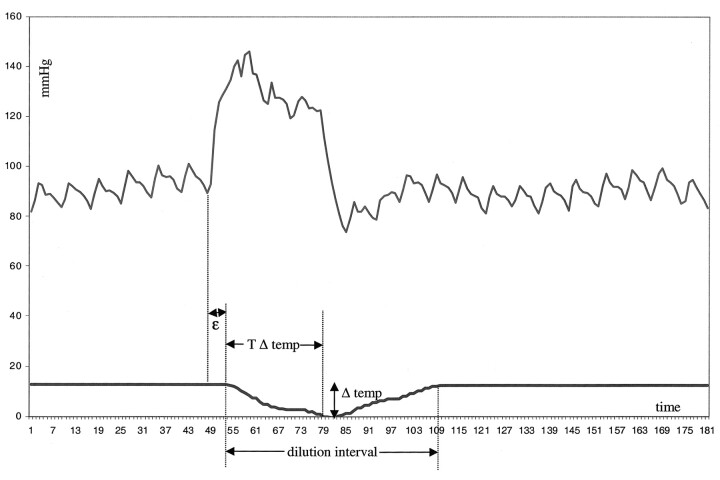
We analyzed the mean pressures before and after GDC placement in both the parent artery and the dome of the aneurysm. Furthermore, we considered the magnitude (Δ temp) and the time interval (T Δ temp) required to obtain the maximum difference in temperature caused by the isotonic sodium chloride injections (Fig 2). The time gap (shift) between the observed increase in pressure after the injection of sodium chloride solution and the start of thermodilution was denoted ε (similar to the phase angle of the vascular input impedance [At positive angles pressure leads flow.]) (18). The total period of temperature change was designated the dilution interval (Fig 2).
Fig 2.
Pressure-thermodilution tracings during an injection of sodium chloride solution over 2 seconds (20 mL/s). At the time of the injection, increase in pressure increases suddenly and quickly; this change is followed by a decrease in temperature. The return of the temperature returns to baseline values is slightly slower than that of pressure. Δ temp indicates the maximum decrease in temperature; T Δ temp, time required to obtain the maximum decrease in temperature; and ε, shift between the observed increases in pressure and flow.
Results
General Observations
The injections of sodium chloride solution created characteristic and reproducible increases in pressure, as well as a specific thermodilution pattern (Fig 2). No vasospasm was observed in response to positioning of the measuring devices or to the sodium chloride injections. No difference was observed in the measurements obtained in the bifurcation aneurysm compared with those in the lateral aneurysms.
Maximum Decrease in Temperature (Δ Temp)
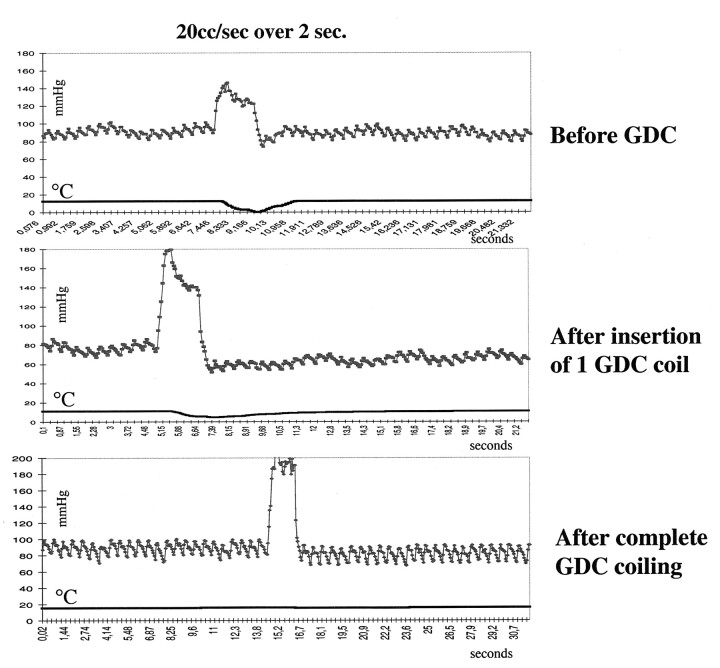
The maximum decrease in temperature was independent of the volume of sodium chloride solution injected (Fig 3). Before coil placement, the response to the sodium chloride solution injection was similar in the parent artery and in the dome of the aneurysm (Tables 1 and 2). Measurements in the aneurysmal domes showed that insertion of GDCs successively diminished the temperature decreases after the sodium chloride solution injections. (Fig 3, Table 1). After coil placement was completed, the decrease in temperature created by the injections of sodium chloride solution was significantly less in the dome of the aneurysm than in the parent artery (P < .05; Wilcoxon signed rank test, two sided). In terms of flow, volume flow in the dome of the aneurysm was reduced to 66% after insertion of one coil. When coil placement was completed, flow was reduced to 30% (range, 0.4–39%) of that before coil placement. In one lateral aneurysm, the maximum difference in the temperature before and after sodium chloride injection was only 0.02°C after complete coil placement; that is, flow had virtually ceased (Fig 3, bottom graph).
Fig 3.
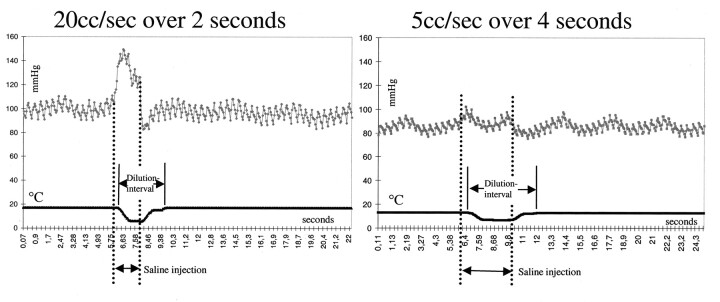
Pressure-thermodilution tracings of injections of sodium chloride solution over 2 seconds (20 mL/s) and 4 seconds (5 mL/s). Higher volumes create a larger increases in pressure but similar decreases in temperature. The length of the dilution interval and pressure increase is dependent on the duration of injection. This finding illustrates the ability to assess flow by using only an injection of only 5 mL/s.
TABLE 1:
Flow characteristics in the dome of the aneurysm before, during, and after GDC placement
| Characteristic | Untreated | One Coil Inserted | P Value after One Coil* | Complete Coil Placement | P Value after Complete Coil Placement* |
|---|---|---|---|---|---|
| Change in temperature (°C) | 10.04 (6.37–12.68) | 6.59 (4.58–6.84) | >.05 | 3.04 (0.02–6.30) | <.05 |
| Time to change in temperature (s) | 1.74 (1.15–2.91) | 2.18 (2.11–3.78) | >.05 | 2.43 (0.86–4.84) | >.05 |
| Time shift ε (s) | 0.45 (0.11–0.53) | 0.34 (0.24–0.44) | >.05 | 0.56 (0.23–2.09) | <.05 |
| Dilution interval (s) | 5.16 (4.17–7.99) | 20.60 (10.32–38.00) | <.05 | 26.79 (9.27–65.48) | <.05 |
Note.—Data are medians. Data in parentheses are ranges.
P values were determined with the Wilcoxon signed rank test (two sided).
TABLE 2:
Flow characteristics in the parent artery before and after GDC placement in the aneurysm
| Characteristic | Untreated | Complete Coil Placement | P Value |
|---|---|---|---|
| Change in temperature (°C) | 11.27 (9.53–11.93) | 7.01 (4.31–8.66) | >.05 |
| Time to change in temperature (s) | 1.65 (0.99–2.47) | 2.12 (1.08–5.07) | >.05 |
| Time shift ε (s) | 0.27 (0.12–0.39) | 0.10 (0.02–0.48) | >.05 |
| Dilution interval (s) | 6.64 (5.12–7.20) | 7.78 (3.85–12.72) | >.05 |
Note.—Data are medians. Data in parentheses are ranges.
P values were determined with the Wilcoxon signed rank test (two sided).
Time Interval to the Maximum Decrease in Temperature (T Δ Temp)
The time required from the injection of sodium chloride solution until the maximum decrease in temperature at the site of measurement was approximately 2 seconds. This time window was the same in the parent artery and in the dome of the aneurysm. It was not significantly affected by GDC placement (Tables 1 and 2).
Pressure-Flow Shift (ε)
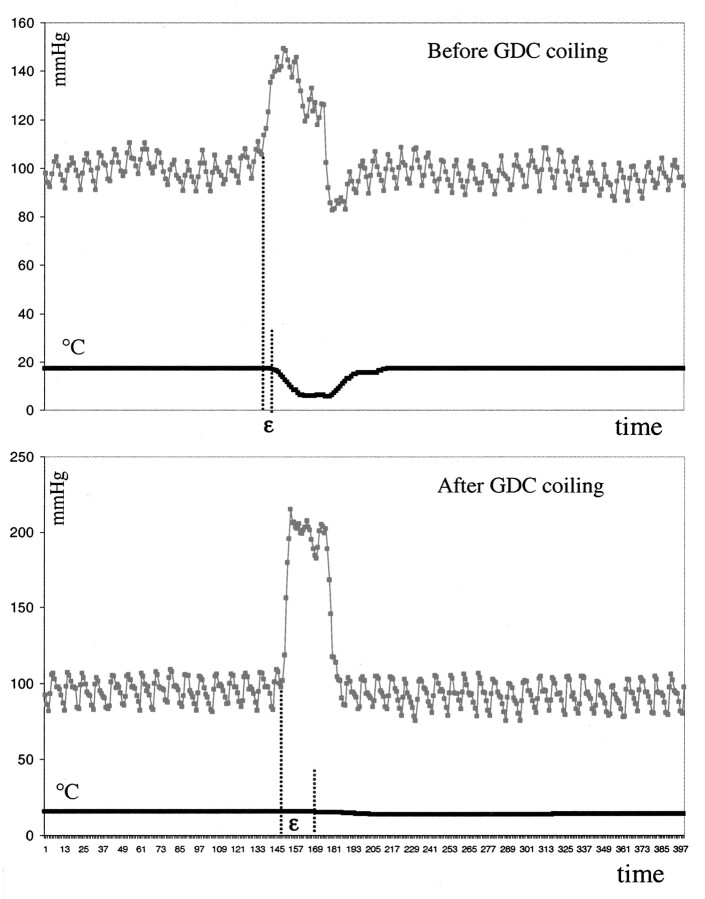
In every instance, pressure led flow. Before treatment, the magnitudes of the shift were similar in the parent artery and in the dome of the aneurysm (Tables 1 and 2). After coil placement, flow was significantly delayed in the dome of the aneurysm (increased shift) but not in the parent artery; that is, a dissociation of pressure and flow occurred in the aneurysm (Fig 4, Tables 1 and 2).
Fig 4.
Pressure-thermodilution tracings of injections of 20 mL/s sodium chloride solution over 2 seconds in the dome of the aneurysm before, during, and after complete GDC placement. The thermodilution trace is markedly flattened; this finding corresponds to a cessation of local blood flow. Pressure is not attenuated. This result illustrates that insertion of only a single coil can dramatically change flow within an aneurysm. Further coil placement in this aneurysm completely interrupted flow in the aneurysm.
Dilution Interval
The dilution interval varied between 4 and 8 seconds both in the parent artery and in the dome of the aneurysm. This was dependent on the duration of the sodium chloride solution injection. The injection of 5 mL/s over 4 seconds created a dilution interval of roughly 8 seconds, and the injection of 20 mL/s over 2 seconds resulted in a dilution interval of approximately 4 seconds (Fig 5). After insertion of the first coil, the dilution interval was considerably prolonged (Fig 3, Table 1). It expanded further after complete coil placement. (Fig 3). Coil placement of the aneurysms did not significantly affect the dilution interval in the parent artery (Table 2).
Fig 5.
Pressure-thermodilution tracings of injections of 20 mL/s sodium chloride solution over 2 seconds in the dome of the aneurysm before and after complete GDC placement demonstrate the resulting dissociation of pressure and flow. Compare the interval between the injection and change in temperature before coil placement to that observed after coil placement.
Discussion
The ability to measure flow by means of thermodilution is derived from the physical principal that the temperature of an injected liquid that flows through a vessel of different temperature changes in proportion to the local rate of flow. Thermodilution is widely used in obtaining cardiac output measurements in critically ill patients; measurements are accurate and reproducible (19). Although iced injectants traditionally have been used for these determinations, recent findings indicate that room-temperature injections of sodium chloride solution are equivalent to the iced injections (20, 21). Because of the advantages of 1) fewer systemic adverse effects triggered by repeated injections of cold sodium chloride solution and 2) reliable and easy zero calibration of the pressure-thermistor wire at room temperature, we choose to use room-temperature injectants in our experiments. Using this technique, we obtained similar and maximal temperature responses with both 5- and 20-mL injections. This finding indicates that an adequate temperature gradient was present between the sodium chloride solution used for injection and canine blood.
The typical thermodilution curve in our experiments was U shaped. After a sodium chloride solution injection (5 or 20 mL) into the parent artery, intravascular temperature successively decreased at the measurement site and reached its lowest point (around room temperature) approximately 2 seconds after completion of the injection (zero calibration, ie, temperature at the thermal sensor corresponds to that of the sodium chloride solution injected). From the lowest point, the temperature then increased to the baseline value; the slope was a mirror image of the slope of the temperature decrease. The shape of the curve was similar for measurements in a parent artery and those in the dome of an aneurysm. This finding indicates that corresponding flow was present at both of these sites. This was not unexpected, because similar blood velocities in the parent artery and in an aneurysm have been described previously (22). Use of a 5-mL injection over 4 seconds for the assessment of changes in flow offers the advantage of allowing multiple measurements without risk of causing unacceptable fluid overload. Coil placement in the aneurysms caused a distortion of the U shape of the thermodilution curve in the dome of the aneurysms, but it did not affect the shape of the curve in the parent artery. Complete coil placement caused a striking change (in one instance, complete flattening) in the thermodilution profile, with flow reductions of 61–99.6%.
Numerous hemodynamic forces affect the formation, growth, and rupture of aneurysms. Although their exact pathophysiologic role in the natural history of saccular aneurysms is not fully understood, the variables of pressure, impulse, and shear stress have been considered to be important in causing aneurysm rupture (23). Since treatment with GDCs leads to an immediate (too early for the occurrence of a biologic response; ie, healing) and dramatic reduction in the incidence of rebleeding in ruptured aneurysms, modification or reduction of one or more of these hemodynamic forces likely is the reason for this protection (5). Under both normotensive and hypertensive conditions, the mean pressure in an aneurysm is not reduced with coil placement (6, 7). However, complete coil placement does attenuate both the amplitude of the pressure and the slope of sudden increases in pressure (8). Thus, the coils seem to exert an effect similar to that of a breakwater. It is unclear if this effect alone is responsible for the observed protective effect of the device or if the coils also induce other hemodynamic changes that provide protection.
To a large degree, shear stress is defined by the volume and velocity of flow. We have demonstrated a dramatic reduction, and even cessation, of the flow rate in the dome of aneurysms after the insertion of GDCs. If the diameter of the aneurysm is assumed to be unchanged immediately after GDC placement, the observed changes in flow should cause a proportional reduction in velocity. Since blood is a non-Newtonian fluid, a decrease in flow and velocity causes an increase in blood viscosity. These changes (decreased flow, decreased velocity, and increased viscosity) create an environment that is favorable to thrombosis. As thrombosis occurs, the changes are amplified so that, ultimately, complete aneurysmal thrombosis may occur (23). Over time, aneurysmal size decreases (24). This mechanism also is likely to be responsible for thrombosis in aneurysms after parent artery occlusion. In this circumstance, flow and velocity decrease distal to the occluded artery.
The inflow into side-wall aneurysms is well described. The blood enters during systole at the downstream ostium, forming a vortex wave, and leaves the aneurysm at the upstream ostium during diastole (13, 15, 25). By changing the morphology of the aneurysm ostium and cavity, insertion of even a single GDC may dramatically alter this predictable flow pattern. Gobin et al (13) described, with their in vitro model, slower filling and washout of the aneurysm after partial coil embolization. Fluid arriving at the coiled aneurysm slows abruptly when it arrived at the first coil and then loses cohesion so that no vortex waves are observed. These findings parallel our observation of a largely increased dilution interval after GDC placement.
Both Boecher-Schwarz et al (7) and Novak et al (6) observed a decrease in aneurysmal wall pulsation after coil placement. This decrease in wall movement after the insertion of either a single coil or multiple coils may occur because of a change in the inflow, and thereby, a decrease in impulse (impulse equals the change in momentum) (23). In oscillating, viscous systems, pressure and flow are not in phase. This means that, when a pulsatile pressure is applied to an elastic chamber (eg, an artery), the flow rate might increase either before or after the time when pressure reaches its maximum. Accordingly, flow leads pressure, and the phase angle of the frequency that is dependent on vascular input impedance is negative (18). We observed this phenomenon in our experiments. Before coil placement, the shifts between pressure and flow were similar in the parent artery and in the aneurysm. After coil placement, the shift increased in the aneurysm. In an ideal system with nonviscous flow and a perfectly elastic tube without any wave reflection, pressure and flow are in phase; that is, they alternate simultaneously. The dissociation of pressure and flow that we observed may reflect changes in both the viscosity of blood and tube elasticity; that is, the rigidity of the aneurysmal wall increased with decreased pulsatile wall excursions.
The reduction in flow after GDC placement had a relatively wide range that did not seem to correlate with the degree of aneurysmal packing. For example, two aneurysms that seemed to be well packed, with similar degrees of angiographic opacification, had different reductions in flow of 61% and 99.6%. Although only five aneurysms were evaluated in our experiments, the changes in flow observed after aneurysmal coil placement, compared with flow in the parent arteries (Tables 1 and 2), give us confidence that our conclusions can be generalized. One might assume that the aneurysm that had a flow reduction of 61% might not trombose completely and, hence, would have a potential for growth or rebleeding. Also, coil compaction might be more likely in an aneurysm with notable residual flow. These observations show that estimation of the completeness of aneurysmal occlusion by means of visual evaluation of angiograms obtained immediately after treatment is not exact and that disturbance of flow created by the coils may be a function of their distribution as much as the packing density. Further efforts to develop techniques for hemodynamic monitoring to guide the neurointerventionalist to the exact point of appropriate coil placement seem to be warranted.
Conclusion
It is possible to evaluate intravascular and intraaneurysmal blood flow by using a 0.014-inch guidewire-mounted thermistor. Blood flows in a parent artery and in the dome of an untreated aneurysm are similar. GDC coil placement in an aneurysm causes a marked reduction or even cessation of flow at the dome. Dissociation of pressure and flow also occurs.
References
- 1.Ferguson GG. Direct measurement of mean and pulsatile blood pressure at operation in human intracranial saccular aneurysms. J Neurosurgery 1972;36:560–563 [DOI] [PubMed] [Google Scholar]
- 2.Sahs AL, Perret GE, Locksley HB, Nishioka H, eds. Intracranial Aneurysms and Subarachnoid Hemorrhage: A Cooperative Study. Philadelphia, Pa: Lippincott,1969
- 3.Crawford T. Some observations on the pathogenesis and natural history of intracranial aneurysms. J Neurol Neurosurg Psychiatry 1959;22:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki J, Ohara H. Clinicopathological study of cerebral aneurysms. J Neurosurg 1978;48:505–514 [DOI] [PubMed] [Google Scholar]
- 5.Graves VB, Strother CM, Duff TA, Perl J. Early treatment of ruptured aneurysm with Guglielmi detachable coils: effect on subsequent bleeding. Neurosurgery 1995;37:640–648 [DOI] [PubMed] [Google Scholar]
- 6.Novak P, Glikstein R, Mohr G. Pulsation-pressure relationship in experimental aneurysms: observation of aneurysmal hysteresis. Neurol Res 1996;18:377–382 [DOI] [PubMed] [Google Scholar]
- 7.Boecher-Schwarz HG, Ringel K, Kopacz L, Heimann A, Kempski O. Ex vivo study of the physical effect of coils on pressure and flow dynamics in experimental aneurysms. AJNR Am J Neuroradiol 2000;21:1532–1536 [PMC free article] [PubMed] [Google Scholar]
- 8.Sorteberg A, Sorteberg W, Turk AS, Rappe A, Nakstad PHJ, Strother LM. Effect of GDC coiling on intra-aneurysmal pressure: an experimental study in canines. AJNR Am J Neuroradiol 2001;22:1750–1756 [PMC free article] [PubMed] [Google Scholar]
- 9.Steiger H, Poll A, Liepsch D, Reulen H, Basic flow structure in saccular aneurysms: a flow visualization study. Heart Vessels 1987;3:55–65 [DOI] [PubMed] [Google Scholar]
- 10.Strother CM, Graves VB, Rappe A. Aneurysm hemodynamics: an experimental study. AJNR Am J Neuroradiol 1992;13:1089–1095 [PMC free article] [PubMed] [Google Scholar]
- 11.Burleson AC, Turitto VT. Identification of quantifiable hemodynamic factors in the assessment of cerebral aneurysm behavior. Thromb Haemost 1996;76:118–123 [PubMed] [Google Scholar]
- 12.Burleson AC, Strother CM, Turitto VT. Computer modeling of intracranial saccular and lateral aneurysms for the study of their hemodynamics. Neurosurgery 1995;37:774–784 [DOI] [PubMed] [Google Scholar]
- 13.Gobin YP, Counord JL, Flaud P, Duffaux J. In vitro study of haemodynamics in a giant saccular aneurysm model: influence of flow dynamics in the parent vessel and effects of coil embolisation. Neuroradiology 1994;36:530–536 [DOI] [PubMed] [Google Scholar]
- 14.German W, Black SPW. Experimental production of carotid aneurysms. N Engl J Med 1954;250:105–106 [DOI] [PubMed] [Google Scholar]
- 15.Graves VB, Strother CM, Partington CR, Rappe A. Flow dynamics of lateral carotid artery aneurysms and their effects on coils and balloons: an experimental study in dogs. AJNR Am J Neuroradiol 1994;13:189–196 [PMC free article] [PubMed] [Google Scholar]
- 16.Leerand S. Thermal flowmeters. In: Cappelen C, ed. New Findings in Blood Flowmetry. Oslo, Norway: Universitetsforlaget;1986;15–17
- 17.Mc Donald. Blood Flow in Arteries. Southampton, England: Camelot Press;1974;222
- 18.Gessner U. Vascular input impedance. In: Bergel DH. Cardiovascular Fluid Dynamics. London, England: Academic Press;1972;315–349
- 19.Kadota LT. Theory and application of thermodilution cardiac output measurement: a review. Heart Lung 1985;14:605–616 [PubMed] [Google Scholar]
- 20.Safcsak K, Nelson LD. Thermodilution right ventricular ejection fraction measurements: room temperature versus cold temperature injectate. Crit Care Med 1994;22:1136–1141 [DOI] [PubMed] [Google Scholar]
- 21.Lehmann KG, Platt MS. Improved accuracy and precision of thermodilution cardiac output measurement using a dual thermistor catheter system. J Am Coll Cardiol 1999;33:883–891 [DOI] [PubMed] [Google Scholar]
- 22.Tenjin H, Asakura F, Ueda S, et al. Evaluation of intraaneurysmal blood velocity by time-density curve analysis and digital subtraction angiography. AJNR Am J Neuroradiol 1998;19:1303–1307 [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson GG. Physical factors in the initiation, growth and rupture of human intracranial saccular aneurysm. J Neurosurgery 1972;37:666–674 [DOI] [PubMed] [Google Scholar]
- 24.Strother CM, Eldevik P, Kikuchi Y, Graves V, Partington C, Merlis A. Thrombus formation and structure and the evolution of mass effect in intracranial aneurysms treated by balloon embolization: emphasis on MR findings. AJNR Am J Neuroradiol 1989;10:787–796 [PMC free article] [PubMed] [Google Scholar]
- 25.Ujiie H, Tachibana H, Hiramatsu O, et al. Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: a possible index for surgical treatment of intracranial aneurysms. Neurosurgery 1999;45:119–129 [DOI] [PubMed] [Google Scholar]