Abstract
Summary: Recanalization of the basilar artery is not always achieved with intraarterial fibrinolysis. We report two cases of thromboaspiration in the basilar artery in which recanalization with fibrinolysis was successful. Thromboaspiration requires favorable anatomy and a fresh nonadhesive clot. It reduces the time for recanalization, has no hemorrhagic risk, and may prevent distal clot migration. Thromboaspiration may be attempted as an adjunct or alternative to intraarterial fibrinolysis for basilar artery recanalization.
Basilar artery occlusion has a poor prognosis (1). Clinical recovery may occur only with early recanalization. Clot dissolution may be attempted with intraarterial fibrinolysis, but this remains unsuccessful in 50% of cases (1, 2). Thromboaspiration is widely used in peripheral arterial thrombus removal (3). The natural tortuosity of the cerebral vessels makes intracranial thromboaspiration difficult. We report two cases of successful recanalization in a basilar artery that was treated with thromboaspiration.
Case Reports
Case 1
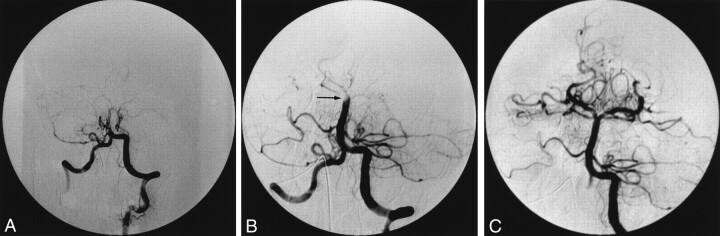
A 37 year-old woman had a progressive alteration of consciousness, with a motor ocular deficit and right hemiparesis. The patient became comatose after 6 hours and was referred to the neuroangiographic suite 20 hours after the first symptoms appeared. All segments of the basilar artery were occluded (Fig 1A). Intraarterial thrombolysis was selected as the treatment, and 1,000,000 U of urokinase were progressively infused. Despite partial thrombus dissolution, the basilar artery remained occluded (Fig 1B). A 4F catheter (Glidecath vertebral curve; Terumo, Japan) was inserted into the vertebral and basilar arteries as far as the remaining thrombus by using a 0.035-inch guidewire (Radiofocus angle type; Terumo, Japan). Aspiration with a 50-mL luer-lock syringe removed all remaining thrombus (Fig 1C). Despite complete recanalization, the clinical outcome was poor, with a locked-in syndrome.
Fig 1.
Case 1. Anteroposterior (AP) angiograms obtained in a 37-year-old woman with a progressive alteration of consciousness, with a motor ocular deficit, right hemiparesis, and eventual coma.
A, View of the posterior circulation after an injection through the left vertebral artery. The proximal basilar artery is occluded.
B, View of the basilar artery after the injection of 1,000,000 U of urokinase. The basilar artery is mostly recanalized. A residual clot (arrow) occludes the basilar bifurcation.
C, View of the basilar artery after thromboaspiration. The basilar artery is permeable after aspiration with a 4F catheter placed at the level of the thrombus.
Case 2
A 3-mm sacciform basilar tip aneurysm was incidentally discovered in a 40-year-old woman (Fig 2B). The patient was referred for endovascular treatment. Usual preventive measures for thromboembolism were used: The guiding catheter (Vista 6F, Cordis; Miami, FL) was proximally placed in the dominant vertebral artery to permit free flow. All catheters were continuously flushed with saline, and 3000 IU of heparin was administered as a bolus, followed by a continuous infusion at a rate of 1500 U/h. After placement of the first GDC coil in the aneurysm failed, a control angiogram was obtained; it showed a large thrombus occluding the distal portion of the left vertebral artery (Fig 2C) and extending into the basilar artery (Fig 2D). Thromboaspiration was attempted as the first treatment. A 5F catheter (Tracker 38; Target) was inserted as far as the origin of the basilar artery by using a 0.035-inch guidewire (Radiofocus; Terumo) (Fig 2E). Two successive aspirations with a 50-mL luer-lock syringe removed most of the thrombus, leaving a small thrombus at the origin of a left common trunk anterior inferior cerebellar artery and posterior inferior cerebellar artery (AICA-PICA) (Fig 2F). After endovascular occlusion of the aneurysm, intraarterial fibrinolysis with 300,000 U of urokinase enabled complete recanalization of the trunk AICA-PICA (Fig 2G). The clinical status was normal at awakening.
Fig 2.
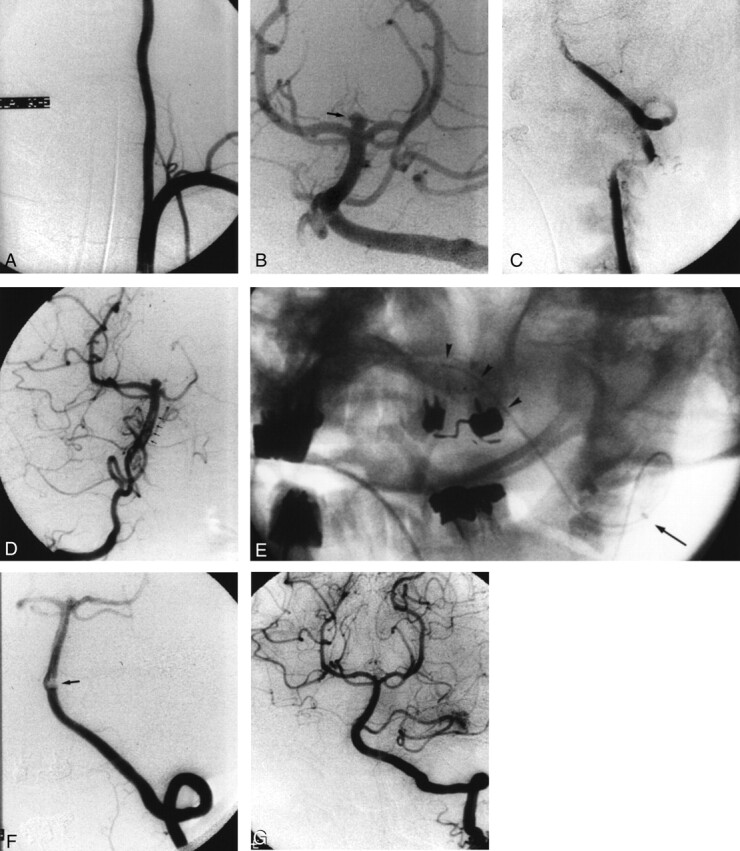
Case 2. Angiograms obtained in a 40-year-old woman with a 3-mm sacciform basilar tip aneurysm who was referred for endovascular treatment.
A, AP view of the dominant left vertebral artery, which has a large diameter and is not tortuous.
B, AP view of the posterior circulation obtained after an injection in the left vertebral artery. A small, incidental, top, basilar aneurysm (arrow) is depicted.
C, AP view of the left vertebral artery obtained after placement of the first coil in the aneurysm failed. The vertebral artery is occluded.
D, AP view of the basilar artery obtained after an injection in the right vertebral artery. The thrombus (arrows) extends from the left vertebral into the basilar artery.
E, Nonsubtracted oblique view obtained during catheterization of the basilar artery. The end of the catheter (arrow) is in the V3 segment, and the guidewire (arrowheads) is in the distal V4 segment of the left vertebral artery.
F, AP view of the left vertebral artery and the posterior circulation after thromboaspiration. A small residual clot (arrow) occludes the origin of the left common AICA-PICA.
G, AP view of the left vertebral artery and the posterior circulation obtained after coil placement in the aneurysm and selective fibrinolysis in the left common trunk AICA-PICA. No residual thrombus is present.
Discussion
The pathogenesis of basilar artery occlusion involves an embolism or local atherothrombosis. In both of our cases, the occlusion resulted from embolism. In case 2, it was a complication of endovascular treatment. Thromboembolic complications during aneurysm embolization are found at angiography in 5.5% (4) to 11% (5) of cases. A higher frequency may be possible; 60% of silent ischemic lesions are found at postembolization MR examination (6). Three mechanisms are possible: embolic migration from the aneurysmal sac, thrombus extension from the aneurysm, and migration from the guiding catheter. Prevention involved the intravenous administration of heparin during the procedure and permanent flushing of dead spaces.
Basilar artery occlusions have a high mortality rate and a poor neurologic outcome (2). Local intraarterial fibrinolysis has a better outcome (7). However, it may not be effective. Furthermore, it may induce intracranial hemorrhage. In case 1, the basilar artery remained occluded despite fibrinolysis; therefore, thromboaspiration was attempted. In case 2, thromboaspiration was attempted as the primary treatment modality rather than intraarterial fibrinolysis. The aneurysm had been manipulated and was untreated when the thromboembolic complication occurred. Aneurysmal manipulation with a coil may cause injury to the aneurysmal wall, and sometimes leads to hemorrhage. In our patient, even if the aneurysm did not bleed before or during embolization, fibrinolysis may have potentiated the risk of hemorrhage. Therefore, we attempted thromboaspiration first to enable basilar recanalization and aneurysmal treatment before adjuvant fibrinolysis.
Thromboaspiration commonly is used in peripheral procedures (3). It has high recanalization rates in occlusions distal to the common femoral artery (8). Thromboaspiration obviously has some advantages: It reduces the time for recanalization, it has no hemorrhagic risk, and it may prevent distal clot migration. Thromboaspiration is more efficient with a fresh nonadhesive clot. Aspiration usually is preceded by high-pressure injections close to the thrombus to liberate it from the wall. This kind of procedure cannot be performed in cerebral vessels because it may induce distal clot migration. Mechanical clot disruption with the use of a microguidewire (9) or balloon angioplasty (10) has been proposed to increase the efficiency of fibrinolysis, but they induce clot fragmentation and distal migration of small thrombi. However, thromboaspiration requires favorable anatomic conditions and a large-lumen catheter. For intracranial use, the catheter must be inserted through tortuous vessels to reach the clot. In both of our cases, insertion of a 4F or 5F catheter to the basilar artery was possible, because the anatomic conformation was very favorable, with a straight and large vertebral artery (Fig 2A). The guidewire and catheter were brought to the base of the thrombus. Caution was taken not to enter the clot with the guidewire to prevent clot disruption and migration. Aspiration was achieved with a 50-mL syringe, which was the largest available syringe that created the highest negative pressure. The catheter was removed after aspiration with the second syringe, in which was suction was maintained during retrieval. The control angiograms were obtained after retrieval and flushing of the catheter to prevent reinjection of the aspirated thrombus. A different catheter was used in each case, according to the availability of large-lumen hydrophilic 4F or 5F catheters. No dedicated device allows intracranial thrombus removal. Forcing the insertion of a large catheter in a less favorable anatomic conformation may induce dissection. Therefore, a large-lumen catheter should be developed specifically for this procedure.
Conclusion
Thromboaspiration in the basilar artery may be attempted in cases with favorable anatomy. The development of specific mechanical recanalization devices should enlarge the field of application.
References
- 1.Hacke W, Zeumer H, Ferbert A, Bruckmann H, del Zoppo GJ. Intraarterial thrombolytic therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke 1988;19:1216–1222 [DOI] [PubMed] [Google Scholar]
- 2.Brandt T, von Kummer R, Muller-Kuppers M, Hacke W. Thrombolytic therapy of acute basilar artery occlusion: variables affecting recanalization and outcome. Stroke 1996;27:875–881 [DOI] [PubMed] [Google Scholar]
- 3.Desgranges P, Kobeiter H, d’Audiffret A, Melliere D, Mathieu D, Becquemin JP. Acute occlusion of popliteal and/or tibial arteries: the value of percutaneous treatment. Eur J Vasc Endovasc Surg 2000;20:138–145 [DOI] [PubMed] [Google Scholar]
- 4.Vinuela F, Duckwiller G, Mawad MJ. Guglielmi detachable coil embolization of acute intracranial aneurysms: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–482 [DOI] [PubMed] [Google Scholar]
- 5.Cognard C, Weill A, Castaings L, Rey A, Moret J. Intracranial berry aneurysms: Angiographic and clinical results after endovascular treatment. Radiology 1998;206:499–510 [DOI] [PubMed] [Google Scholar]
- 6.Rordorf G, Bellon RJ, Budzik RF, et al. Silent thromboembolic events associated with the treatment of unruptured cerebral aneurysms by use of Guglielmi detachable coils: prospective study applying diffusion-weighted imaging. AJNR Am J Neuroradiol 2001;22:5–10 [PMC free article] [PubMed] [Google Scholar]
- 7.Berg-Dammer E, Felber SR, Henkes H, Nahser HC, Kuhne D. Long-term outcome after local intraarterial fibrinolysis of basilar artery thrombosis. Cerebrovasc Dis 2000;10:183–188 [DOI] [PubMed] [Google Scholar]
- 8.Starck EE, McDermott JC, Crummy AB, Turnipseed WD, Acher CW, Burgess JH. Percutaneous aspiration thromboembolectomy. Radiology 1985;156:61–66 [DOI] [PubMed] [Google Scholar]
- 9.Connors JJ. Strategic considerations concerning emergency stroke treatment. In: Connors JJ, Wojak JC, eds. Interventional Neuroradiology: Strategies and Practical Techniques. Philadelphia, Pa: WB Saunders;1999;650–692
- 10.Cognard C, Weill A, Lindgren S, Piotin M, Castaings L, Moret J. Basilar artery occlusion in a child: “clot angioplasty” followed by thrombolysis. Childs Nerv Syst 2000;16:496–500 [DOI] [PubMed] [Google Scholar]