Abstract
BACKGROUND AND PURPOSE: The perceived safety of the recreational drug methylenedioxymethamphetamine (MDMA), or Ecstasy, conflicts with animal evidence indicating that MDMA damages cortical serotonin (5-HT) neurons at doses similar to those used by humans. Few data are available about the effects of MDMA on the human brain. This study was designed to evaluate MDMA-related alterations in metabolite ratios with single-voxel proton (1H) MR spectroscopy.
METHODS: Fifteen male MDMA users (mean lifetime exposure, 723 tablets; mean time since last tablet, 12.0 weeks) and 12 age-matched control subjects underwent single-voxel 1H MR spectroscopy. N-Acetylaspartate (NAA)/creatine (Cr), NAA/Choline (Cho), and myoinositol (MI)/Cr ratios were measured in midfrontal gray matter, midoccipital gray matter, and right parietal white matter. Data were analyzed with linear model-based multivariate analysis of variance.
RESULTS: NAA/Cr (P = .04) and NAA/Cho (P = .03) ratios, markers associated with neuronal loss or dysfunction, were reduced in the frontal cortex of MDMA users. Neither NAA/Cr (P = .72) nor NAA/Cho (P = .12) ratios were different between both groups in occipital gray matter and parietal white matter (P = .18). Extent of previous MDMA use and frontal cortical NAA/Cr (ρ = −.50, P = .012) or NAA/Cho (ρ = −.550, P < .01) ratios were significantly associated.
CONCLUSION: Reduced NAA/Cr and NAA/Cho ratios at 1H MR spectroscopy provide evidence for neuronal abnormality in the frontal cortex of MDMA users; these are correlated with the degree of MDMA exposure. These data suggest that MDMA may be a neurotoxin in humans, as it is in animals.
It has become increasingly apparent that the popular recreational drug 3,4-methylenedioxymethamphetamine (MDMA), Ecstasy, can lead to toxic effects in brain serotonin (5-HT) neurons in animals and, possibly, humans. In animals, damage to 5-HT neurons has been demonstrated with reductions in various markers that are unique to 5-HT axons, including brain 5-HT, 5-hydroxyindoleacetic acid (5-HIAA), and the density of 5-HT transporters (1–5). Anatomic studies in MDMA-treated animals indicate that these neurochemical changes are secondary to a distal axonotomy of 5-HT neurons (6, 7).
Although good evidence for a neurotoxic potential of MDMA in animals exists, studies of the potential neurotoxic effects of MDMA in humans are lacking. A few groups have evaluated CSF 5-HIAA concentrations in MDMA users and found either normal (8) or decreased levels (9, 10). Neuroendocrine challenge tests have been used; however, to our knowledge, they have not been validated for the detection of neuronal injury (11). Recent brain imaging studies have been conducted to investigate the effect of MDMA on human 5-HT neurons with either positron emission tomography (PET) or single-photon emission CT (SPECT) (12–15). These investigators reported alterations in the density of presynaptic 5-HT transporters in MDMA users that were similar to those observed in MDMA-treated rodents and nonhuman primates. These changes affected 5-HT-rich brain regions such as the hypothalamus and cortical gray matter, whereas cortical white matter was relatively unaffected (11).
These observations, which are suggestive of neurotoxicity in human MDMA users, prompt further investigation of the possible neurotoxic effects of MDMA in the human brain, particularly because the perceived safety of MDMA conflicts with animal evidence of MDMA neurotoxicity and because of the increasing prevalence of hazardous patterns of recreational use (16). For instance, a recent report (17) suggests that the number of episodes involving the intake of multiple tablets is increasing. Furthermore, MDMA often is used in environments that are hot and crowded in which access to drinking water is limited; these conditions increase the risk of hyperthermia, which exacerbates MDMA neurotoxicity in rats (18).
Proton (1H) MR spectroscopy of the brain is a noninvasive study of certain aspects of cerebral biochemistry. Peaks of N-acetyl groups (primarily N-acetylaspartate [NAA]), choline (Cho)-containing compounds, and creatine (Cr) plus phosphocreatine are present in the spectrum. Also, determination of the myoinositol (MI) peak is possible with short TEs. NAA is contained almost exclusively within neuronal cell bodies and axons (19), and it is considered a marker for neuronal loss or dysfunction (20, 21). Commonly, NAA/Cr and NAA/Cho ratios are determined, and this practice seems valid because NAA/Cr and NAA/Cho ratios are reduced in a variety of brain diseases associated with neuronal loss.
The present study was designed to evaluate whether MDMA use leads to alterations in metabolite ratios—in particular, NAA/Cr and NAA/Cho ratios—at single-voxel 1H MR spectroscopy. MDMA is known to induce 5-HT neurotoxicity in the cortical gray matter of animals, while leaving cortical white matter relatively unaffected. Therefore, we hypothesized that, compared with control subjects, MDMA users have lower NAA/Cr and/or NAA/Cho ratios in gray matter brain regions but not in white matter brain regions.
Methods
Participants
MDMA users were compared with ecstasy-naïve but drug-using control subjects. Subjects were recruited with flyers distributed at venues associated with the “rave scene,” with the help of an agency that provides harm-reduction information and advice. We reduced limiting factors associated with potential preexisting differences and the use of other drugs by recruiting controls from the same population as ecstasy users. This design is conspicuously different from that of most previous studies, in which control subjects were recruited from a university or general population. Control subjects were group-matched for sex and age (18–45 years) and were otherwise healthy. The eligibility criterion for the MDMA group was previous use of a minimum of 50 tablets of Ecstasy. The control subjects were healthy and had no self-reported prior use of Ecstasy. All participants completed a detailed drug history questionnaire. Participants agreed to abstain from the use of psychoactive drugs for at least 1 week before the study and were asked to undergo urine drug screening (with an enzyme-multiplied immunoassay for amphetamines, barbiturates, benzodiazepine metabolites, cocaine and its metabolite, opiates, and marijuana) before enrolment. Exclusion criteria were the following: a positive finding at drug screening, pregnancy, and severe medical or neuropsychiatric illness that precluded the acquisition of informed consent. Subjects were interviewed with a structured automated diagnostic method (Composite International Diagnostic Interview [CIDI], version 2.1) to screen for current axis I psychiatric diagnoses. The institutional medical ethics committee approved the study. After we completely described the study to the patients and subjects, written informed consent was obtained from all participants.
MR Spectroscopy
Brain 1H MRS was performed with a 1.5-T Signa Echo Speed unit (GE Medical Systems, Milwaukee, WI) by using the standard quadrature head coil. The MR protocol consisted of multisection sagittal and coronal fast spin-echo proton density- and T2-weighted imaging (TR/TE, 4000/22–97; section thickness, 5 mm; field of view, 23 cm; matrix, 256 × 256).
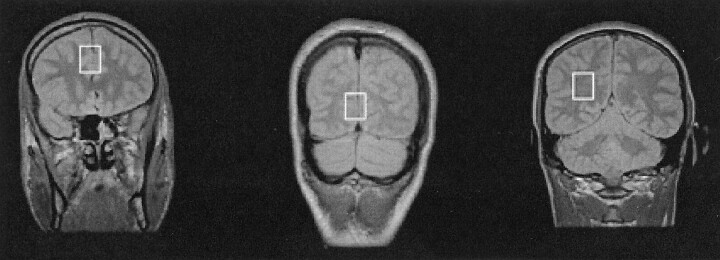
The 1H spectra were collected from three brain regions: midfrontal gray matter, midoccipital gray matter, and right parietal white matter (Fig 1). The voxel size was 4.5 cm3 (15 × 15 × 20 mm), and voxels were chosen carefully to ensure that each contained primarily gray matter or white matter. Data were acquired by using a fully automated execution of proton brain examination (PROBE) provided by the manufacturer of the MR machine. The point-resolved spectroscopic (PRESS) sequence was chosen for its known robustness (22) and optimized for the chosen TEs and locations (3000/35, 128 signals acquired, 2.5-kHz bandwidth). The spectroscopic data acquisition provided a water-suppressed proton spectrum over a range from 4.3 to −0.5 ppm. The pure metabolic signal was apodized, zero filled, and Fourier transformed to produce the spectrum. The numeric analysis was based on peak amplitude by normalizing the linewidths of the peaks. This analysis allowed effective measurement of the areas and ratio of areas (23). All peaks in the designated spectral area were curve fitted by using a Marquardt fitting routine. Because absolute measures of 1H MRS metabolites are influenced by various technical parameters, including transmitter gain and receiver settings, MR spectroscopic signals commonly are measured as ratios of one metabolite to another. NAA/Cr, NAA/Cho, and MI/Cr ratios were calculated.
Fig 1.
Proton density-weighted MR image (4000/22) shows the three voxel locations for the localized 1H MRS studies: midfrontal gray matter (left), mid-occipital gray matter (middle), and right parietal white matter (right).
Statistical Analysis
Comparisons between MDMA users and control subjects for descriptive variables such as age, verbal intelligence, and recreational exposure to drugs other than MDMA were performed by using two-tailed unpaired Student t tests. The main effect of MDMA use on metabolite ratios (NAA/Cr, NAA/Cho, and MI/Cr) was studied by using a general linear model-based multivariate analysis of variance (MANOVA) in which possible correlations between the brain regions studied and multiple comparisons were taken into account. Gray matter (frontal and occipital cortex) and white matter (parietal) were analyzed separately. Age and extent of previous cannabis and amphetamine use were covariates. If MANOVA revealed a significant effect, we investigated the differences in metabolite ratios between the groups by using one-way analysis of variance (ANOVA). Since the use of methamphetamine has been associated with reductions in NAA levels in the basal ganglia and frontal white matter of MDMA users (24), the data also were analyzed after we excluded those subjects who indicated that they used amphetamines in addition to MDMA in the 3 months before the study. (The use of methamphetamine is uncommon in the Netherlands, unlike, for instance, in the Unites States.) The a priori postulated correlation between NAA levels and extent of previous MDMA use and duration of abstinence was assessed by using the Spearman correlation coefficient. The chance of a type I error (α) was set at 0.05. All data were analyzed by using SPSS (version 9.0; SPSS Software, Chicago, IL).
Results
The two groups were similar in age. Recreational use of alcohol and tobacco was comparable between MDMA users and control subjects. MDMA users indicated that they used more cannabis and amphetamines in the 3 months before this investigation compared with controls. This difference was significant for the extent of previous cannabis use (P = .006) but not for the extent of amphetamine use (P = .18) (Table 1). Four of 15 MDMA users indicated that they used amphetamines in the 3 months before the study.
TABLE 1:
Demographic data and characteristics of MDMA users and control subjects
| Characteristic | Control Subjects(N = 12) | MDMA Users(N = 15) |
|---|---|---|
| Age (y) | 27.0 ± 4.1 | 27.2 ± 5.3 |
| MDMA use | ||
| Duration (y) | NA | 5.6 (2.5–12.0) |
| Usual dose (no. of tablets) | NA | 2.1 (1.5–3.5) |
| Lifetime dose (no. of tablets) | NA | 723 (55–2176) |
| Time since last tablet (wk) | NA | 12.0 (1–40) |
| Other drugs | ||
| Alcohol (units per wk) | 13.4 ± 11.9 | 17.5 ± 13.8 |
| Tobacco (no. of cigarettes per day) | 10.8 ± 3.7 | 13.3 ± 14.9 |
| Cannabis (no. of joints in the last 3 mo) | 2.3 ± 0.5 | 158.3 ± 178.9* |
| Amphetamine (no. of uses in the last 3 mo) | NA | 5.0 ± 8.7 |
Note.—Data are the means ± SDs or means (ranges). NA indicates not applicable.
The difference between MDMA users and control subjects was statistically significant (P < .01, unpaired Student t test).
In the MDMA group, participants generally had used more than 700 tablets over a period of 2–3 years. Most of the MDMA users had not used MDMA for weeks, and some indicated that they had not used MDMA for several months (Table 1).
MR Findings
Visually, no notable brain atrophy or white matter lesions were detected on the images in either MDMA users or control subjects.
1H MRS Findings
A significant mean group effect was observed in the two gray matter brain regions studied (F = 2.82, df = 6.0, P = .045) but not in the white matter region (F = 1.79, df = 3.0, P = .180). The covariance effects of age, extent of previous cannabis use, and extent of previous amphetamine use were not significant (P = .73, P = .77, and P = .14, respectively) in terms of the significant mean group effect observed in the gray matter. ANOVA demonstrated that NAA/Cr and NAA/Cho ratios in the frontal gray matter of MDMA users were significantly lower compared with those of control subjects (P = .04 and P = .03, respectively), but ratios in occipital gray matter were not (P = .72 and P = .12, respectively). Results are summarized in Table 2 and Figure 2. No lactate or excess lipid levels were observed in any of the spectra. When amphetamine users were excluded from the analysis, NAA/Cr and NAA/Cho ratios in the frontal cortex still were significantly lower in MDMA users compared with control subjects (P = .004 and P = .046, respectively).
TABLE 2:
1H MRS findings in gray and white matter regions in MDMA users and control subjects
| Voxel Location | Control Subjects* (N = 12) | MDMA Users* (N = 15) | Difference (%) | P Value† |
|---|---|---|---|---|
| Gray matter ratios‡ | ||||
| Midfrontal | ||||
| NAA/Cr | 1.62 ± 0.20 | 1.43 ± 0.21 | −11.7 | .04 |
| NAA/Cho | 2.06 ± 0.23 | 1.78 ± 0.27 | −14.0 | .03 |
| MI/Cr | 0.65 ± 0.08 | 0.64 ± 0.06 | −1.5 | .62 |
| Midoccipital | ||||
| NAA/Cr | 1.56 ± 0.19 | 1.54 ± 0.21 | −0.1 | .72 |
| NAA/Cho | 3.04 ± 0.52 | 2.67 ± 0.61 | −12.2 | .12 |
| MI/Cr | 0.60 ± 0.07 | 0.57 ± 0.08 | −5.0 | .29 |
| Right parietal white matter ratios§ | ||||
| NAA/Cr | 1.90 ± 0.10 | 1.78 ± 0.19 | −6.3 | NA |
| NAA/Cho | 1.84 ± 0.16 | 1.96 ± 0.32 | +6.5 | NA |
| MI/Cr | 0.64 ± 0.06 | 0.62 ± 0.09 | −3.1 | NA |
Data are the means ± SDs.
NA indicates not applicable.
At MANOVA, F = 2.82, df = 6.0, P = .045.
At MANOVA, F = 1.79, df = 3.0, P = .180.
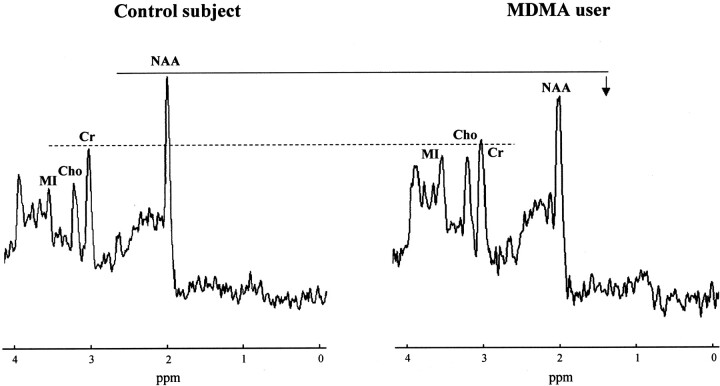
Fig 2.
Proton MRS spectra from the midfrontal gray matter region in a control subject (age, 23 years) and an MDMA user (cumulative lifetime exposure, 200 tablets; age, 26 years). The spectrum in the MDMA user is representative for the whole group and shows a reduction in NAA levels, with Cr levels similar to those in the control subject. Note that MI levels are elevated in this specific MDMA user, although for the group as a whole, mean MI measures in MDMA users did not statistically differ from those of control subjects.
No significant correlations were observed between metabolite ratios and duration of abstinence from MDMA. However, a significant association was observed between the extent of previous MDMA use (log transformed) and NAA/Cr (ρ = − .50, P = .012) and NAA/Cho (ρ = − .550, P = .003 [Fig 3]) ratios in the frontal cortex. The higher the MDMA exposure, the lower NAA/Cr and NAA/Cho ratios.
Fig 3.
Plot shows the correlation between the NAA/Cr ratio in the frontal cortex and the extent of previous MDMA use.
Discussion
In the present study, we observed reductions in NAA/Cr and NAA/Cho ratios in the frontal gray matter of MDMA users, and we found that decreases in NAA/Cr and NAA/Cho ratios are usage related.
NAA is a correlate marker for healthy mature neurons (20); therefore, a reduction in NAA levels indicates reduced neuronal density. NAA levels are reduced in a number of pathologic processes that affect the integrity of neurons (25, 26). For instance, neurotoxic damage to dopaminergic neurons induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, or MPTP, results in persistent decreases in NAA levels in the monkey brain (25). Reductions in NAA levels may be reversed with treatment in some instances (27–29).
Taken in conjunction with previous results that show selective decreases in concentrations of CSF 5-HIAA (9, 10) and 5-HT transporter densities in MDMA users (12–15) and with similar findings in MDMA-treated animals with documented neurotoxic lesions (1, 3, 6, 11, 30), these data provide further evidence that human MDMA users may be susceptible to MDMA-induced neuronal abnormality. In support of this possibility are the presently observed dose-dependent decreases in NAA/Cr and NAA/Cho ratios in MDMA users. However, in view of the small brain mass occupied by 5-HT nerve terminals (eg, ≪1%), MDMA-induced gross loss of 5-HT neurons in the prefrontal cortex is not likely the cause of the observed reductions in NAA levels of around 13%. Rather, they reflect nonspecific neuronal loss or dysfunction of neurons in the prefrontal cortex of MDMA users.
We observed reductions in NAA/Cr and NAA/Cho ratios in frontal gray matter but not in occipital gray matter or right parietal white matter. This finding was in agreement with our hypothesis, with the exception that no significant reductions in NAA levels were observed in the occipital gray matter. Regional differences in the neurotoxic effects of MDMA within the gray matter of the cortex have been shown, but they seem to vary with the technique used. For instance, reductions in regional brain levels of 5-HT or 5-HIAA (as measured with high-performance liquid chromatography with electrochemical detection, or HPLC-EC) were examined in an MDMA-treated baboon, the occipital cortex was the brain region most affected (11). However, in that same baboon, [3H]paroxetine-labeled 5-HT transporters were reduced by 85% in the frontal and parietal cortex, whereas in the temporal and occipital cortex, transporter densities were reduced by 73% and 78%, respectively.
Consistent with the present findings, reductions in NAA concentrations recently have been reported in users of methamphetamine, a compound with actions similar to those of MDMA (24). In this study, NAA concentrations in the frontal white matter and basal ganglia of methamphetamine users were significantly reduced compared with those of control subjects. Methamphetamine has been shown to be neurotoxic to both dopaminergic and serotonergic neurons in rodents. Recent PET studies in abstinent methamphetamine users have revealed reductions in dopaminergic terminals similar to those reported in methamphetamine-treated nonhuman primates (31, 32).
In one other study (33), 1H MRS was used to investigate the effects of MDMA on brain neurochemistry. In this study, no difference in the NAA/CR ratios of MDMA users and control subjects was observed. Discrepancies between the present findings and those of Chang et al (33) may be attributed, in part, to the fact that the subjects in our study had used nearly six times as much MDMA: The cumulative lifetime exposure to MDMA was, on average, 131 tablets in the study by Chang (based on a dose of 100 mg MDMA per tablet or capsule), whereas the MDMA users in our study had an average exposure of 723 tablets.
Apart from the observed reductions in NAA/Cr and NAA/Cho ratios in the frontal cortex of MDMA users, no metabolite abnormalities were observed in the present study. In particular, we did not observe significant alterations in MI/Cr ratios in MDMA users, although in some users, elevated MI/Cr ratios were observed (Fig 3). In contrast, Chang and coworkers (33) previously reported a significant increase in MI and MI/Cr levels in the parietal white matter of MDMA users. MI is believed to be located primarily in glial cells and absent in neurons. Elevations may be attributed to gliosis and reactive astrocytosis. However, normal aging has been shown to increase MI levels (34, 35). Discrepancies between the findings by Chang and those of the present study may be attributed, in part, to age-associated differences. In the present study, participants (both MDMA users and control subjects) were younger and had a smaller age range (median age of MDMA users 26.0 years ± 5.3; range, 20–38 years), whereas the median age of MDMA users in the study by Chang et al was 43.0 years ± 14.6, with an age range of 19–75 years. However, precise quantification of near-water resonance peaks, such as that of MI at 3.56 ppm, is difficult at water-suppressed 1H MR spectroscopy. This problem also may account for the discrepancy between the findings.
Although NAA is found almost exclusively within neurons (20), the exact functional implications of reduced NAA levels remain unclear. In addition to serving as a storage form of aspartate, NAA is in the metabolic pathway for glutamate, and although it is not thought to be a neurotransmitter per se (36), it is capable of inducing calcium influx by means of N-methyl-d-aspartic acid receptors in vitro (37). Interestingly, a recent study revealed increased NAA measures in rats during experimental status epilepticus; this finding suggests that NAA levels are correlated with the functional status of neurons. Similarly, NAA levels in the prefrontal cortex of patients with schizophrenia have been shown to be strongly correlated with activation during a working memory task (38). Furthermore, we recently reported a strong association between delayed memory function and NAA/Cr ratios in the prefrontal cortex of Ecstasy users (39).
Several potential limitations of the current study should be mentioned. First, as with all retrospective studies, preexisting differences between MDMA users and controls may underlie the differences in NAA concentrations. For instance, critics have argued that low 5-HT function may be a cause, rather than an effect, of MDMA use, because low concentrations of 5-HT have been linked to impulsivity and sensation seeking in humans (40). However, since NAA, to our knowledge, has not been linked to impulsivity and sensation seeking, the present study makes it less probable that observed decreases in NAA/Cr and NAA/Cho are a cause rather than an effect of MDMA use. Second, because most of the MDMA users had experimented with other recreational drugs, mainly cannabis and amphetamines, we cannot completely rule out the possibility that the present findings are unrelated to cannabis or amphetamine use. However, urine drug test results indicated that no participant had used cannabis in the week before the study. While, to our knowledge, the effects of cannabis use on NAA levels are still unknown, methamphetamine has been shown to reduce NAA levels (24). In the present study, when MDMA users who had used amphetamine in the 3 months before the study were excluded from the analysis, NAA/Cr and NAA/Cho ratios in the frontal cortex still were significantly lower in MDMA users than those in control subjects. Also, no significant effect of cannabis or amphetamine use on NAA/Cr and NAA/Cho ratios in the frontal cortex was observed in the statistical analysis; this result makes it less probable that the findings of the present study should be attributed to the use of either of these drugs. In addition, observed decreases in NAA/Cr and NAA/Cho ratios are unlikely to be due to direct pharmacologic effects of MDMA, because MDMA users reported that they had abstained from the use of MDMA or other psychoactive drugs for at least 1 week before the study. Furthermore, we had to rely on retrospective accounts of drug-use histories from the drug history questionnaire.
A recent survey was conducted to investigate the validity of the drug history questionnaire used in this study. It revealed that, in 93% of the cases, the reported use of Ecstasy agreed with the results of urine drug testing (41). However, periods of drug usage and abstinence also were verified with urine drug screening. Blood and urine samples can be used to detect drugs such as cannabis 2–3 weeks after use, but MDMA and other amphetamine derivatives can be detected only 24–48 hours after the last dose. Therefore, we were able to objectively confirm abstention from cannabis use, not MDMA use, in the 2–3 weeks before the study. In future studies, hair-sample analysis may be useful and more appropriate to ascertain previous MDMA use and to determine what drug was taken and at what time it was taken. Finally, single-voxel proton spectra were acquired and processed automatically in this study. The standard variations in NAA/Cr ratios have been shown to be much lower with this method that with studies that rely on manual processing or manual adjustment of the instrument or both. Regarding the remaining variability, how much is controllable, and how much is caused by biologic variation in the healthy population? One potential source of error is the partial volume effect. Contamination of the volume of interest with different tissue types leads to variations in the results. In future studies, this problem may be corrected with tissue segmentation to identify gray matter and white matter fractions. NAA/Cr ratios are known to be higher in white matter than in gray matter. Therefore, contamination of a gray voxel with white matter increases the NAA/Cr ratios, and consequently, underestimation of neurotoxic effects of MDMA on 5-HT neurons occurs, because MDMA is known to induce 5-HT neurotoxicity in cortical gray matter and leave cortical white matter relatively unaffected (11).
Conclusion
Our results suggest that MDMA use may lead to changes in NAA levels in the frontal cortex of MDMA users, as measured with 1H MRS, and that these changes are dose related. These findings confirm and extend previous observations, suggesting that human MDMA users may be at risk of neuronal injury. The present findings provide a cogent argument for consumers to make informed decisions about recreational MDMA use. Additional studies are needed to determine whether changes in NAA concentrations in MDMA users are reversible with longer periods of abstinence and whether reduced NAA levels are associated with functional impairments.
Footnotes
Presented in part at the 86th scientific assembly and annual meeting of the Radiological Society of North America, Chicago, IL, November, 2000.
References
- 1.Schmidt CJ. Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J Pharmacol Exp Ther 1987;240:1–7 [PubMed] [Google Scholar]
- 2.Stone DM, Stahl DC, Hanson GR, Gibb JW. The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur J Pharmacol 1986;128:41–48 [DOI] [PubMed] [Google Scholar]
- 3.Battaglia G, Yeh SY, O’Hearn E, Molliver ME, Kuhar MJ, De Souza EB. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labeled serotonin uptake sites. J Pharmacol Exp Ther 1987;242:911–916 [PubMed] [Google Scholar]
- 4.Ricaurte GA, Forno LS, Wilson MA, et al. (+/−)3,4-Methylenedioxymethamphetamine selectively damages central serotonergic neurons in nonhuman primates. JAMA 1988;260:51–55 [PubMed] [Google Scholar]
- 5.Ricaurte GA, Martello AL, Katz JL, Martello MB. Lasting effects of (+−)-3,4-methylenedioxymethamphetamine (MDMA) on central serotonergic neurons in nonhuman primates: neurochemical observations. J Pharmacol Exp Ther 1992;261:616–622 [PubMed] [Google Scholar]
- 6.O’Hearn E, Battaglia G, De Souza EB, Kuhar MJ, Molliver ME. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J Neurosci 1988;8:2788–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson MA, Ricaurte GA, Molliver ME. Distinct morphologic classes of serotonergic axons in primates exhibit differential vulnerability to the psychotropic drug 3,4-methylenedioxymethamphetamine. Neuroscience 1989;28:121–137 [DOI] [PubMed] [Google Scholar]
- 8.Peroutka SJ. Incidence of recreational use of 3,4-methylenedimethoxymethamphetamine (MDMA, “ecstasy”) on an undergraduate campus. N Engl J Med 1987;317:1542–1543 [DOI] [PubMed] [Google Scholar]
- 9.McCann UD, Ridenour A, Shaham Y, Ricaurte GA. Serotonin neurotoxicity after (+/−)3,4-methylenedioxymethamphetamine (MDMA; “Ecstasy”): a controlled study in humans. Neuropsychopharmacology 1994;10:129–138 [DOI] [PubMed] [Google Scholar]
- 10.Ricaurte GA, Finnegan KT, Irwin I, Langston JW. Aminergic metabolites in cerebrospinal fluid of humans previously exposed to MDMA: preliminary observations. Ann N Y Acad Sci 1990;600:699–708 [DOI] [PubMed] [Google Scholar]
- 11.Scheffel U, Szabo Z, Mathews WB, et al. In vivo detection of short- and long-term MDMA neurotoxicity–a positron emission tomography study in the living baboon brain. Synapse 1998;29:183–192 [DOI] [PubMed] [Google Scholar]
- 12.McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet 1998;352:1433–1437 [DOI] [PubMed] [Google Scholar]
- 13.Semple DM, Ebmeier KP, Glabus MF, O’Carroll RE, Johnstone EC. Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (‘ecstasy’) users. Br J Psychiatry 1999;175:63–69 [DOI] [PubMed] [Google Scholar]
- 14.Reneman L, Booij J, de Bruin K, et al. Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA. Lancet 2001;358:1864–1869 [DOI] [PubMed] [Google Scholar]
- 15.Reneman L, Reneman L, Lavalaye J, et al. Cortical serotonin transporter density and verbal memory in individuals who stopped using 3,4-methylenedioxymethamphetamine (MDMA or “Ecstasy”): preliminary findings. Arch Gen Psych 2001;58:901–906 [DOI] [PubMed] [Google Scholar]
- 16.Boot BP, McGregor IS, Hall W. MDMA (Ecstasy) neurotoxicity: assessing and communicating the risks. Lancet 2000;355:1818–1821 [DOI] [PubMed] [Google Scholar]
- 17.Topp L, Hando J, Dillon P, Roche A, Solowij N. Ecstasy use in Australia: patterns of use and associated harm. Drug Alcohol Depend 1999;55:105–115 [DOI] [PubMed] [Google Scholar]
- 18.Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci 1998;18:5086–5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe FA, Maxwell RJ, Saunders DE, Brown MM, Griffiths JR. Proton spectroscopy in vivo. Magn Reson Q 1993;9:31–59 [PubMed] [Google Scholar]
- 20.Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 1993;13:981–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higuchi T, Graham SH, Fernandez EJ, et al. Effects of severe global ischemia on N-acetylaspartate and other metabolites in the rat brain. Magn Reson Med 1997;37:851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci 1987;508:333–348 [DOI] [PubMed] [Google Scholar]
- 23.Webb PG, Sailasuta N, Kohler SJ, Raidy T, Moats RA, Hurd RE. Automated single-voxel proton MRS: technical development and multisite verification. Magn Reson Med 1994;31:365–373 [DOI] [PubMed] [Google Scholar]
- 24.Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology 2000;54:1344–1349 [DOI] [PubMed] [Google Scholar]
- 25.Brownell AL, Jenkins BG, Elmaleh DR, Deacon TW, Spealman RD, Isacson O. Combined PET/MRS brain studies show dynamic and long-term physiological changes in a primate model of Parkinson disease. Nat Med 1998;4:1308–1312 [DOI] [PubMed] [Google Scholar]
- 26.Arnold DL, Riess GT, Matthews PM, et al. Use of proton magnetic resonance spectroscopy for monitoring disease progression in multiple sclerosis. Ann Neurol 1994;36:76–82 [DOI] [PubMed] [Google Scholar]
- 27.Cendes F, Andermann F, Dubeau F, Matthews PM, Arnold DL. Normalization of neuronal metabolic dysfunction after surgery for temporal lobe epilepsy: evidence from proton MR spectroscopic imaging. Neurology 1997;49:1525–1533 [DOI] [PubMed] [Google Scholar]
- 28.De Stefano N, Matthews PM, Antel JP, Preul M, Francis G, Arnold DL. Chemical pathology of acute demyelinating lesions and its correlation with disability. Ann Neurol 1995;38:901–909 [DOI] [PubMed] [Google Scholar]
- 29.Vion-Dury J, Nicoli F, Salvan AM, Confort-Gouny S, Dhiver C, Cozzone PJ. Reversal of brain metabolic alterations with zidovudine detected by proton localised magnetic resonance spectroscopy. Lancet 1995;345:60–61 [DOI] [PubMed] [Google Scholar]
- 30.Hatzidimitriou G, McCann UD, Ricaurte GA. Altered serotonin innervation patterns in the forebrain of monkeys treated with (+/−)3,4-methylenedioxymethamphetamine seven years previously: factors influencing abnormal recovery. J Neurosci 1999;19:5096–5107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci 1998;18:8417–8422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villemagne V, Yuan J, Wong DF, et al. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci 1998;18:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang L, Ernst T, Grob CS, Poland RE. Cerebral (1)H MRS alterations in recreational 3, 4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users. J Magn Reson Imaging 1999;10:521–526 [DOI] [PubMed] [Google Scholar]
- 34.Chang L, Ernst T, Poland RE, Jenden DJ. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci 1996;58:2049–2056 [DOI] [PubMed] [Google Scholar]
- 35.Fowler JS, Volkow ND, Wang GJ, et al. Age-related increases in brain monoamine oxidase B in living healthy human subjects. Neurobiol Aging 1997;18:431–435 [DOI] [PubMed] [Google Scholar]
- 36.Tsai G, Coyle JT. N-acetylaspartate in neuropsychiatric disorders. Prog Neurobiol 1995;46:531–540 [DOI] [PubMed] [Google Scholar]
- 37.Rubin Y, LaPlaca MC, Smith DH, Thibault LE, Lenkinski RE. The effect of N-acetylaspartate on the intracellular free calcium concentration in NTera2-neurons. Neurosci Lett 1995;198:209–212 [DOI] [PubMed] [Google Scholar]
- 38.Bertolino A, Esposito G, Callicott JH, et al. Specific relationship between prefrontal neuronal N-acetylaspartate and activation of the working memory cortical network in schizophrenia. Am J Psychiatry 2000;157:26–33 [DOI] [PubMed] [Google Scholar]
- 39.Reneman L, Majoie CBLM, Schmand B, van den Brink W, den Heeten GJ. Prefrontal N-acetylaspartate is strongly associated with memory performance in (abstinent) Ecstasy users: preliminary report. Biol Psych 2001;50:550–554 [DOI] [PubMed] [Google Scholar]
- 40.Reed LJ, Winstock A, Cleare AJ, McGuire P. Toxic effect of MDMA on brain serotonin neurons. Lancet 1999;353:1268–1271 [DOI] [PubMed] [Google Scholar]
- 41.Van de Wijngaart G, Braam R, De Bruin D, Fris M, Maalsté N, Verbraeck H. Ecstasy in het uitgaanscircuit (Ecstasy and the Dutch Rave Scene: A Socio-epidemiological Study on the Nature and Extent of, and the Risks Involved in using Ecstasy and Other Party Drugs at Dance Events). Utrecht, the Netherlands: Addiction Research Institute;1997