Abstract
Summary: We describe the case of a 54-year-old woman with a clinical diagnosis of Churg-Strauss syndrome (CSS). The patient had a fever of unknown origin, severe headache, progressing left ophthalmoplegia, and visual acuity disturbance. MR imaging revealed diffuse and thick hypointense lesions on T2-weighted images in the frontal meninges and anterior falx cerebri with diffuse enhancement. Similar lesions were also detected in the left superior ophthalmic fissure to the cavernous sinus. Nodular lesions in the fourth ventricle, which might have been the cause of hydrocephalus, were hypointense on T2-weighted images. These MR imaging findings suggested remote granulomatous involvement in the meninges and choroid plexus associated with CSS. To our knowledge, remote meningeal involvement in association with CSS has not been previously reported.
The Churg-Strauss syndrome (CSS) is characterized by disseminated vasculitis in patients with asthma and consists of a constellation of symptoms, including fever, hypereosinophilia, and multisystem involvement. Characteristic histopathologic findings are necrotizing arteritis or venulitis, eosinophilic infiltration, and extravascular granulomas. Few reports of CNS involvement in cases of CSS exist in the literature. We present a case in which MR imaging revealed granulomatous formation in the meninges, choroid plexus in the fourth ventricles, and cavernous sinus. To our knowledge, this is the first report of meningeal and choroid plexus involvement in a patient with CSS.
Case Report
A 54-year-old woman was admitted to our hospital with a fever of unknown origin, severe headache, progressive ophthalmoplegia on the left, and visual acuity disturbance. She had been examined for recurrent rhinitis and bronchial asthma at age 48 years. At that time, laboratory studies revealed a high erythrocyte sedimentation rate (110 mm/h) and leukocytosis (13,800 WBC/mm3) with 23% eosinophils.
The serum level of perinuclear antineutrophil cytoplasmic antibody (ANCA), determined by using the enzyme-linked immunosorbent assay method, was positive at 30 enzyme-linked immunosorbent assay units (normal, <10 enzyme-linked immunosorbent assay units), and serum had negative findings for the cytoplasmic type of ANCA. Chest radiography revealed no infiltration. Bone marrow biopsy revealed normocellular bone marrow with eosinophilia. Kidney biopsy showed vasculitis of the small- to medium-sized vessels with eosinophilic infiltration; clinicopathologically, this case included all the diagnostic criteria for CSS proposed by the American College of Rheumatology in 1990 (1). The elevated serum titer of perinuclear ANCA further supported the diagnosis (2).
MR imaging performed at 1.5 T with a Vision system (Siemens, Erlangen, Germany) revealed focal thickening and enhancement of the anterior falx cerebri and meninges, especially in the frontal region (Fig 1H). These thickened meninges were hypointense on T2- (3500/120 [TR/TE]) and T1-weighted (600/15) images. A similar lesion was also noted in the left cavernous sinus that extended to the ipsilateral superior ophthalmic fissure and temporal meninges. Nodular hypointense lesions, which might have caused hydrocephalus, were noted in the fourth ventricle on T2-weighted images. These showed enhancement on T1-weighted images.
Fig 1.
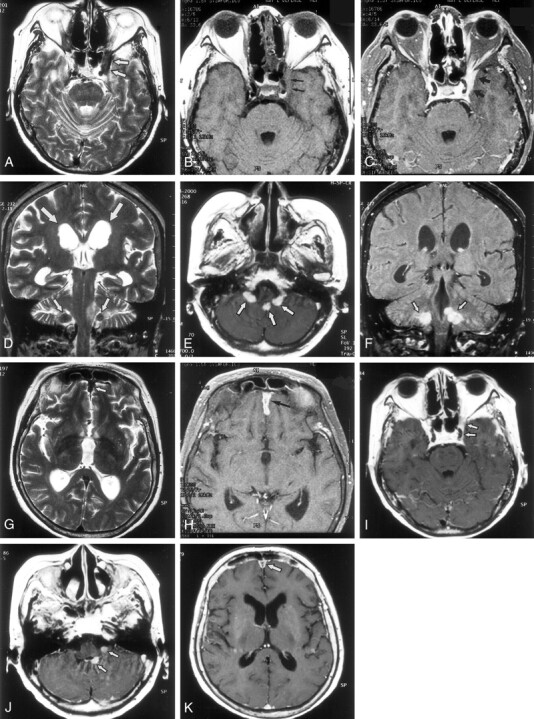
MR findings in a 54-year-old woman with CSS.
A, Axial T2-weighted image (3500/120/2 [TR/TE/excitations]) shows a hypointense plaquelike lesion (arrows) involving dura of the left cavernous sinus and superior ophthalmic fissure.
B, Axial T1-weighted image (600/15/1) shows that the lesion (arrows) is isointense to brain.
C, Axial contrast-enhanced T1-weighted image (600/15/1) shows abnormal enhancement of the lesion (arrows) around the left cavernous sinus and the superior ophthalmic fissure.
D, Coronal T2-weighted image (3500/120/2) shows hypointense nodules (small arrows) in the bilateral lateral recess of the fourth ventricle. Dilatation of the lateral ventricle is shown (large arrows).
E, Axial contrast-enhanced T1-weighted image (600/15/1) shows enhancing nodular lesions in the fourth ventricle, extending to bilateral lateral recess (arrows).
F, Coronal contrast-enhanced T1-weighted image (600/15/1) shows enhancing nodular lesions (arrows) in the fourth ventricle that extend to the bilateral lateral recess.
G, Axial T2-weighted image (3500/120/2) shows hypointense lesion (arrow) along the anterior falx cerebri.
H, Axial contrast-enhanced T1-weighted image (600/15/1) shows thickened enhancement along the anterior falx cerebri (arrow).
I, Axial contrast-enhanced T1-weighted image (600/15/1), obtained 9 months after treatment with steroids and immunosuppressants, shows reduction of the abnormal enhancing lesions around the left cavernous sinus and superior ophthalmic fissure (arrows).
J, Axial contrast-enhanced image (600/15/1), obtained at the same time as that shown in panel I, shows nodular lesions (arrows) smaller than those at treatment.
K, Axial contrast-enhanced image (600/15/1), obtained at the same time as those shown in panels I and J, shows a decrease in degree of enhancement along the falx cerebri (arrow) and improvement of the hydrocephalus.
Laboratory studies revealed leukocytosis (13,200 WBC/mm3) without a high rate of eosinophilia. The serum level of C-reactive protein was 5.6 mg/dL (normal, <0.5 mg/dL), and perinuclear ANCA results were positive at 10 enzyme-linked immunosorbent assay units. The cell count in the CSF was 38/3 without any atypia. Serum and CSF specimens did not show any parasites, fungi, or high viral antibody titers (including anti-human immunodeficiency virus).
On the basis of MR imaging findings and clinical history, a diagnosis of remote granulomatous involvement of the meninges and choroid plexus associated with CSS was made. Treatment with steroids and immunosuppressants was instituted, and follow-up MR imaging performed 9 months after admission revealed a marked improvement of clinical and imaging findings.
Discussion
The patient was a 54-year-old woman with a history of asthma and eosinophilia who presented with severe headache, progressive ophthalmoplegia, and vision loss. T2-weighted images depicted hypointense lesions that showed enhancement of the left superior ophthalmic fissure, the cavernous sinus, and the dura of the temporal base, which suggested that these lesions were responsible for progressive ophthalmoplegia and visual acuity disturbance. Similar lesions expanded the meninx of the frontal lobe and falx cerebri. Nodular lesions were hypointense, and enhancement was observed on either side of the fourth ventricle, consistent with the choroid plexus, which might have produced hydrocephalus. Meningeal infiltration and hydrocephalus may have been the causes of the headache that was difficult to control. Owing to the clinical history of the patient, hypointensity of the lesions on T2-weighted images, and continuing remission by treatment with steroids and immunosuppressants, granulomatous meningeal and choroid plexus involvements in CSS were most likely. Pachymeningitis caused by fungal infection, sarcoidosis, and malignant lymphoma were excluded as diagnoses on the basis of CSF cytologic and culture results and the clinical course.
CNS system involvement has been reported to be a complication in 6.3–27.0% of CSS cases (3–5). Many clinical manifestations, such as disorientation, convulsions, and coma, have been cited. Psychiatric disturbances and encephalopathy in CSS are generally transient and improve with the subsiding clinical course or are considered to be nonspecific symptoms of preterminal events. Cerebrovascular events have been reported, with a frequency of 6.2–6.4% (3, 5). Fewer reports of cerebrovascular events have been presented with imaging or clear pathologic findings (6, 7).
Sehgal et al (3) investigated the neurologic manifestations of this disorder and found that cerebrovascular abnormalities occurred in only three (6.4%) of 47 patients with CSS, and all three of these patients had cerebral infarcts. Kok et al (8) reported findings of multiple small infarctions in the subcortical white matter in a child with CSS. Cerebral hemorrhage, frequently resulting in death, has also been found in patients with CSS. Fatal intraventricular and subarachnoid hemorrhages have been reported in a patient with pathologically proved CSS. Liou et al (6) reported multiple cerebral hemorrhages in both hemispheres in a patient with CSS. The exact causes of CSS-associated intracranial hemorrhage remain to be elucidated, but a case of fatal intraventricular hemorrhage and subarachnoid hemorrhage was reported with vasculitis that involved the choroid plexus. In addition to vasculitis, several reports have indicated a close relationship between hypertension and cerebral hemorrhage in patients with CSS. Hypertension is a common finding in association with CSS that may reflect renal and autonomic dysfunction.
Our literature review of cases of intracranial involvement in CSS yielded no reports of patients who had granuloma in the meninx and choroid plexus; to our knowledge, this is the first report. It has been suggested that thorough examination of regions proximal to the meninx by MR imaging, including contrast-enhanced imaging, may elucidate the lesion responsible for cranial neuropathy and ophthalmic involvement, which have been considered to have unknown causes. Granuloma also involved the choroid plexus in the fourth ventricle and may have been the cause of hydrocephalus.
Diseases with disseminated vasculitis similar to CSS include Wegener granulomatosis and microscopic polyarteritis nodosa. Meningeal involvement was reported in 2–8% of cases of Wegener granulomatosis (9–11). According to Murphy et al (12), meningeal enhancement was observed in 11 of 19 patients and was not contiguous to the paranasal sinus lesion in six patients. Autopsy reports of 104 cases of Wegener granulomatosis, presented by Drachman (13), showed that meningeal involvement by a necrotic and granulomatous process was found in one case. As described previously, patients with CSS in whom vasculitis developed in the choroid plexus have been reported. These reports indicate that granuloma formation in the meninx and choroid plexus in cases of CSS is possible.
A specific clinical test for vasculitis is lacking, and a definite diagnosis can be difficult to make in many cases. The classification of vasculitis often is not clear and, in the past, has been confusing. However, ANCAs were identified as autoantibodies, and a new classification system of vasculitis based on the presence or absence of ANCA autoantibodies has been developed (14). Vasculitis occurs in all types of blood vessels, including the aorta to large- and medium-sized arteries, capillary blood vessels, and veins. Vasculitis has been classified on the basis of size of the affected blood vessels as well as clinical characteristics. ANCA-associated vasculitis also has been used to classify subcategories of small- and large-vessel vasculitis. ANCA-associated vasculitis includes small-vessel vasculitis, such as Wegener granulomatosis, CSS, and microscopic polyarteritis nodosa. Large-vessel vasculitis, such as Takayasu disease, is classified as non–ANCA-associated vasculitis. The presence or absence of ANCA adds specificity to the previous classification scheme that is based on histopathologic findings and clinical features. For CNS involvement in Wegener granulomatosis, CSS, and microscopic polyarteritis nodosa, the differences and consistencies of findings should be compared as a type of ANCA-associated vasculitis. This patient had positive findings for perinuclear ANCA when she had clear respiratory symptoms. Although changes in the ANCA titer were not correlated with the course of the intracranial lesions in this patient, the ANCA titer was reported to have diagnostic value and utility as an index of disease activity, therapeutic effect, exacerbation, and recurrence (15, 16). A comparison with future images is desired.
References
- 1.Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angitis). Arthritis Rheum 1990;33:1094–1100 [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ. Clinical and pathological classification of ANCA-associated vasculitis: what are the controversies? Clin Exp Immunol 1995;101(suppl 1):18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sehgal M, Swanson JW, DeRemee RA, Colby TV. Neurological manifestations of Churg-Strauss syndrome. Mayo Clin Proc 1995;70:337–341 [DOI] [PubMed] [Google Scholar]
- 4.Lanham JG, Elkon KB, Pusey CD, Hughes GR. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine 1984;63:65–81 [DOI] [PubMed] [Google Scholar]
- 5.Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome clinical study and long-term follow-up of 96 patients. Medicine 1999;78:26–37 [DOI] [PubMed] [Google Scholar]
- 6.Liou HH, Liu HM, Chiang IP, Yeh TS, Chen RC. Churg-Strauss syndrome presented as multiple intracerebral hemorrhage. Lupus 1997;6:279–282 [DOI] [PubMed] [Google Scholar]
- 7.Chang Y, Kargas SA, Goates JJ, Horoupian DS. Intraventricular and subarachnoid hemorrhage resulting from necrotizing vasculitis of the choroid plexus in a patient with Churg-Strauss syndrome. Clin Neuropathol 1993;12:84–87 [PubMed] [Google Scholar]
- 8.Kok J, Bosseray A, Brion JP, Micoud M, Besson G. Chorea in a child with Churg-Strauss syndrome. Stroke 1993;24:1263–1264 [DOI] [PubMed] [Google Scholar]
- 9.Hoffman GS, Kerr GS, Leavitt RY. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992;16:488–498 [DOI] [PubMed] [Google Scholar]
- 10.Fausi AS, Haynes BF, Katz P, Wolff SM. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 1983;98:76–85 [DOI] [PubMed] [Google Scholar]
- 11.Nishino H, Rubino FA, DeRemee RA, Swanson JW, Parisi JE. Neurological involvement in Wegener’s granulomatosis: an analysis of 324 patients at the Mayo Clinic. Ann Neurol 1993;33:4–9 [DOI] [PubMed] [Google Scholar]
- 12.Murphy JM, Gomez-Anson B, Gillard JH, et al. Wegener granulomatosis: MR imaging findings in brain and meninges. Radiology 1999;213:794–799 [DOI] [PubMed] [Google Scholar]
- 13.Drachman DA. Neurological complications of Wegener’s granulomatosis. Arch Neurol 1963;8:145–155 [Google Scholar]
- 14.Gross WL, Schmitt WH, Csernok E. ANCA and associated diseases: immunodiagnostic and pathogenetic aspects. Clin Exp Immunol 1993;91:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brijker F, Magee CC, Tervaert JW, O’Neill S, Walshe JJ. Outcome analysis of patients with vasculitis associated with antineutrophil cytoplasmic antibodies. Clin Nephrol 1999;52:344–351 [PubMed] [Google Scholar]
- 16.Cohen Tervaert JW, Huitema MG, Hene RJ, et al. Prevention of relapses in Wegener’s granulomatosis by treatment based on antineutrophil cytoplasmic antibody titre. Lancet 1990;336:709–711 [DOI] [PubMed] [Google Scholar]


