Abstract
Summary: Curvilinear reformatting of 3D MR imaging data sets was used to visualize the position of subdural strip and grid electrodes relative to the underlying cerebral cortex in patients with epilepsy who were undergoing invasive electroencephalographic recordings. The contour of the cortical surface was delineated interactively, and topographical relationships among surface gyration, cortical lesions, and subdural electrodes were investigated by using serial convex planes parallel to the cortical surface. Electrode contacts could be marked and their positions projected to underlying areas at different depths. This method is apt for routine purposes and allows electrode positions to be displayed with respect to cortical and subcortical regions of interest.
In the presurgical evaluation of patients with focal epilepsy, invasive electroencephalographic (EEG) recordings have remained necessary for subgroups of patients in whom surface electrodes do not identify the seizure-onset zone with sufficient certainty or precision (1–3). For this purpose, either subdural multicontact electrodes or intracerebral depth electrodes can be used, depending on the topography of the area to be recorded. Subdural electrodes are placed by means of burr holes (strip electrodes) or by craniotomy (grid electrodes) (4). Grid electrodes offer the additional opportunity for mapping eloquent areas of the cortex in patients in whom a resection in that vicinity is taken into consideration.
For a proper interpretation of EEG recordings of the irritative zone and seizure-onset zone, and of data obtained with functional mapping by using electrical cortical stimulation, knowledge concerning the position of intracranial electrodes relative to the cortical surface is essential. So far, the position of subdural electrodes has been estimated from postimplantation skull radiographs and planar 2D MR imaging sections. Recently, improvements with 3D reconstructions of 3D MR imaging and spiral CT data and with intraoperative digital photography of the cortical surface and grid have been reported (5, 6). Whereas the former method requires the acquisition of two 3D data sets and considerable time for image fusion, the latter method can be applied only to patients in whom craniotomy is performed to place electrodes over the lateral cerebral convexity.
We describe the application of curvilinear reformatting of single 3D MR imaging data sets for quick visualization of subdural strip and grid electrodes in relation to underlying cortical gyration and cortical or subcortical lesions.
Technique and Patients
MR Imaging Data Acquisition
The 3D MR imaging data sets were acquired by using a magnetization-prepared rapid acquisition gradient-echo sequence (9.7/4/1 [TR/TE/excitations]; flip angle, 12°) with a 1.5-T unit (Magnetom Vision; Siemens, Erlangen, Germany). The following parameters were used to obtain isotropic voxels of 1 mm2: matrix, 256 × 256; sections, 160–180; field of view, 256 mm; 3D slab thickness, 160–180 mm. Data sets were transferred in digital imaging and communications in medicine (DICOM) format to a G3 Power Macintosh workstation and further processed by using Brainsight software (Rogue Research, Montreal, Quebec, Canada).
Curvilinear Reformatting
The method of curvilinear multiplanar reformatting of 3D MR imaging data sets is described in detail elsewhere (7). In brief, the surface contour of the hemispheric convexities, including the subdural electrodes, was delineated interactively at 10–15 positions placed on the top of the cortical gyri or in the center of electrode contacts on coronal or axial planar sections. Then, curved surfaces 1 mm thick were automatically generated at different depths that included the central position of the electrodes, superficial gyri and sulci, and, in some patients, lesions in greater depth. A series of four to 10 surfaces of progressive depths were displayed from the same angle of view either simultaneously (in as many as six windows) or in a peel mode, allowing a rapid switch between surfaces at several depths (Brainsight; Rogue Research). Electrodes of particular interest were marked interactively; this procedure allowed their positions to be projected into underlying surfaces of greater depth and the topographical relationship of individual grid or strip positions to the cortical surface and to lesions to be established. Distances between points of interest (eg, between the planned resection border and anterior tip of the temporal lobe) could be displayed interactively.
Patients
MR imaging data sets from 27 patients (18 male, nine female; mean age, 30.0 years; age range, 15–60 years) with epilepsy in whom subdural strips and/or grid electrodes were implanted from July 1999 to November 2000 at the University of Freiburg Epilepsy Unit were used for curvilinear 3D reformatting. Strip and grid electrodes consisted of four to 64 stainless steel contacts with interelectrode distances of 10–15 mm embedded in silastic (Ad-Tech, Racine, WI). Subdural strip electrodes were placed over the lateral convexity, interhemispherically, or below the basal surface of the frontal or temporal lobe by means of burr holes. For grid placement, craniotomy with opening of the dura was performed over the area to be recorded. Electrode wires were tunneled subcutaneously for a distance of 2–3 cm to prevent infections (8). No complications occurred during implantation, EEG monitoring, or explantation.
Results
The time required for acquisition of 3D MR imaging data sets was 8–10 minutes, depending on head size and number of section partitions. Delineation of the cerebral surface lasted for another 10–15 minutes. An additional 1–15 minutes was required for marking specific electrodes. The automatic generation of curved surfaces involved 2–5 minutes of data processing.
In each patient evaluated, all electrode contacts could be visualized, and the spatial relationship to anatomic and abnormal structures could be depicted. For the interpretation of EEG data, the identification of the positions of individual electrode contacts often remained difficult when planar MR imaging sections were used alone. Because of the convexity of the cortical surface and the overlying subdural electrodes, planar MR images often displayed only few contacts. Thus, it was necessary to sequentially examine series of MR imaging sections, and even then it was only possible to get a rough overall impression of the relation between the positions of different strip electrodes and the underlying cortex. In contrast, curvilinear reformatting of 3D MR imaging data sets displayed the relationship between subdural electrodes and cortical landmarks (eg, the sylvian fissure or central sulcus) on a single image (Fig 1). Severe bending of electrodes was particularly prominent when they were placed infratemporally or over the temporal or frontal pole. In these patients, it sometimes was impossible to follow the course of a single strip electrode on planar MR imaging sections and to identify the individual position of an electrode contact with respect to the underlying cortex. Again, curvilinear reformatting of 3D MR imaging data sets allowed the individual contacts and their position to be displayed, thus rendering possible a tailored resection of the epileptogenic zone.
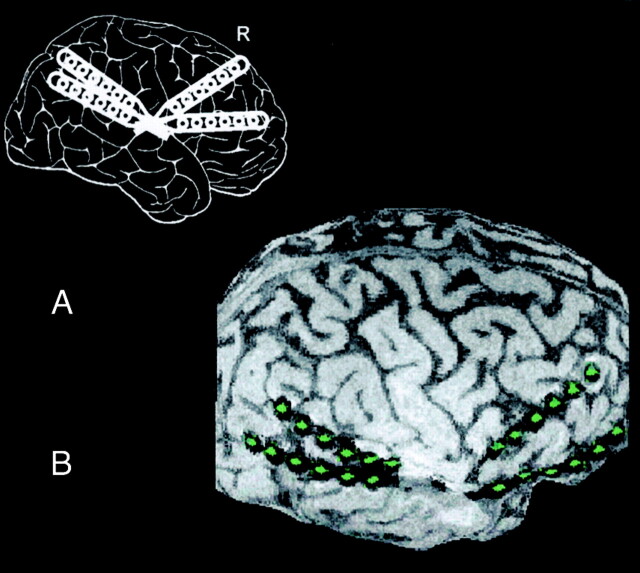
Fig 1.
Positions of subdural strip electrodes in a 16-year-old male adolescent with pharmacorefractory focal epilepsy resulting from tuberous sclerosis. Invasive recordings were necessary to identify the electrophysiologically leading tuber of three tubers shown on MR images. Recordings showed a seizure-onset zone over the right temporal tuber with rapid spread to the frontal cortex; surgical excision of the temporal tuber based on the recordings rendered the patient seizure free.
A, Schematic displays the planned implantation of subdural strip electrodes. R indicates right.
B, Lateral curvilinear reformation shows the cortical surface with four overlying subdural strip electrodes consisting of six electrode contacts each (green). Whereas planar MR imaging sections (not shown) were difficult to interpret because of the underlying convexity of the cortical surface, the curvilinear reformation clearly depicts the spatial relationships between electrodes and the sylvian fissure and cortical gyrus.
Aside from displaying the positions of electrodes with regard to the surface contour, it might be important to clarify their relation to lesions in the depth of the brain. Planar MR imaging with a fixed orientation of sections was often insufficient to display electrode contacts in relation to deep lesions. Even when the orientation of sections was freely selected from 3D MR imaging data sets, the identification of individual electrode contacts and their projection to an underlying lesion remained cumbersome and, to some degree, uncertain. The use of curvilinear reformatting allowed individual electrode positions to be displayed with regard to the underlying lesion, even when situated deeply (Figs 2 and 3). This was particularly important for patients in whom eloquent cortical areas were close to the epileptogenic zone to be resected, by allowing for an exact delineation of the surgical resection, taking into consideration the results of cortical mapping and EEG recordings.
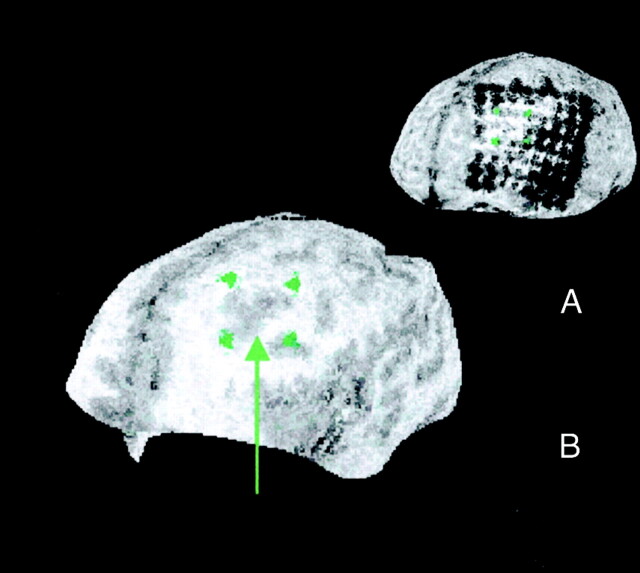
Fig 2.
Curvilinear reformations show relative position of subdural electrode contacts to a cortical dysplasia located deep in the left frontal lobe. In this 23-year-old man with pharmacoresistant focal epilepsy with hypermotor and right tonic seizures from his first year of life, an 8 × 8 subdural grid electrode was implanted over the left frontal cortex for identification of the seizure-onset zone and mapping of language and motor cortex. As mapping of the speech area using electrical stimulation showed a wide extension of the language area, which corresponded to extended activated areas at functional MR imaging during language tasks, the relative position to an underlying suspected cortical dysplasia was crucial for the possibility of removing the lesion without language deficit. Identification of the electrode contacts overlying the dysplasia was based on the results of curvilinear reformatting of 3D MR imaging data. They were found to be part of both the seizure-onset zone and language area. Because of the possible risk of aphasia with a resection of the seizure-onset zone, surgery was deferred in this patient.
A, Projection of grid positions to the underlying cortical dysplasia at a depth of 20 mm.
B, Selected grid positions are indicated by green markers, and the dysplastic cortex showing blurred gray matter–white matter junction is indicated by a green arrow.
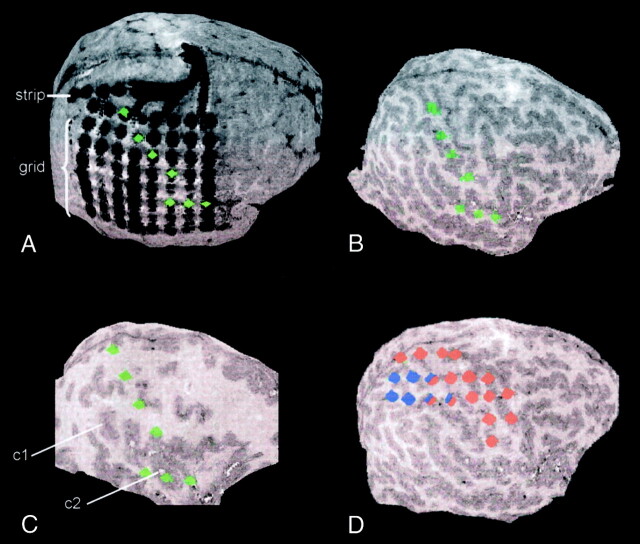
Fig 3.
Curvilinear reformations show electrode positions of a 64-contact subdural grid and a parasagittally placed six-contact-strip electrode. Subdural recordings were performed in this 12-year-old patient with an extended cortical dysplasia including the right supramarginal gyrus to delineate the borders of the irritative zone and seizure-onset zone and for functional mapping of the sensory and motor strip. On planar MR imaging sections (not shown), the spatial relationship between electrode positions and the central sulcus could not be depicted clearly, and the relationship between the subdural strip electrode and the grid electrode remained uncertain. Curvilinear reformatting provided exact information about the relative positions of strip and grid electrodes, as well as the spatial relationship of grid electrode positions and the central sulcus, sylvian fissure, and dysplastic cortex. This was used to plan functional mapping of the somatosensory and somatomotor cortex by electrical stimulation and to plan the resection, which rendered the patient seizure free without persistent neurologic deficits.
A, At a depth of 1 mm, the relative positions of strip and grid electrodes are displayed. Green markers indicate the electrode contacts overlying the postcentral gyrus and the sylvian fissure.
B, At a depth of 10 mm, the relative position of the anatomically marked electrodes (green) and cortical gyration is shown.
C, At a depth of 22 mm, thickening of the cortex along the supramarginal gyrus (c1) and around the superior temporal sulcus (c2) is prominent. Again, the relative position of the dysplastic areas to the anatomically marked electrode contacts (green) is shown.
D, Markers display the results of electrocorticographical mapping of the motor (red) and sensory (blue) strip.
Discussion
Curvilinear multiplanar reformatting of 3D MR imaging data sets has been shown to be superior to conventional planar MR imaging sections in the identification of cortical dysplasias (7). The present investigation shows its usefulness in displaying the topographical relation among subdurally placed electrodes, cortical superficial and deep lesions, and landmarks of the underlying cerebral cortex (9).
Conventional skull radiographs are often used to show the positions of implanted subdural electrodes. However, they do not display the structure of the underlying brain, and the number of different views is restricted. Thus, they provide only gross information about the position and orientation of subdural electrodes and their wiring.
Direct inference of electrode positions from conventional 2D MR imaging sections is often difficult and does not give a good overview of the position of strip and grid electrodes, because only a small fraction of electrode contacts can be visualized on single-plane images. Thus, the subsequent inspection of many sections is necessary to obtain a global impression of the strip or grid electrode positions. Cortical landmarks often are difficult to identify simultaneously with the electrodes. Problems especially occur in areas where electrodes are heavily bent along the convexity of the underlying cortical surface.
The fusion of MR imaging and CT data sets and subsequent 3D reconstructions as described by Winkler et al (5) and similar methods described by other authors (10, 11) can, in principle, yield all necessary data for a realistic modeling of electrode positions. However, it requires the subsequent acquisition of two data sets, and data processing is time consuming.
Compared with these methods, curvilinear reformatting can be used with the acquisition of a single 3D MR imaging data set, and it is much quicker to apply (ie, within 30 minutes vs at least half a day with the method by Winkler et al). Thus, it can be used for routine purposes. Curvilinear reformatting allows cortical surface and electrode positions to be displayed simultaneously and, thus, provides important information of sufficient spatial resolution to be used for cortical mapping and for planning of surgery. It gives a better overview of the relative positions of electrodes and landmarks of the neocortex than do planar MR imaging sections. Furthermore, it is ideally suited for establishing the relative positions with regard to lesions below the surface, in particular cortical dysplasias, by allowing the cortical anatomy at its surface and at different depths below to be visualized. Finally, curvilinear reformatting enables neurosurgeons to view the surface from a similar angle of view, as they do during removal of cortical tissue or subpial transections.
Intraoperative photography (6) is another method proposed recently for localizing electrode positions in relation to the underlying cortex. It can be applied quickly and provides exact localizing information about the position of individual electrode contacts of subdural grids relative to the underlying cortex. Intraoperative photography has a high spatial resolution and offers the advantage that cortical veins also can be taken into consideration as landmarks by the neurosurgeon. With our method, cortical veins cannot be visualized without application of contrast enhancement during acquisition of 3D MR imaging data sets. The use of photography is, however, restricted to patients in whom craniotomy is performed for electrode placement (ie, mainly grid electrodes) and to patients with electrodes placed over the lateral convexity of the brain. In contrast, curvilinear reformatting of 3D MR imaging data sets also allows the display of grid electrodes placed on the basal surface of the cortex interhemispherically and subdural strip electrodes placed by means of burr holes (in which deviations between planned and real positions of implanted electrodes are greatest because they are placed without view of the cortical surface).
Conclusion
Curvilinear reformatting of T1-weighted 3D MR imaging data sets acquired after implantation of subdural strip and grid electrodes can be performed with few hardware requirements and within a time frame that allows its routine use. It provides a quick overview of the position of subdural electrodes with respect to cortical landmarks, even if electrodes are bent and difficult to visualize on conventional orthogonal sections. In addition, the relationship of electrodes to cortical lesions can be shown, and projections of electrode positions to lesions situated deep in the neocortex can be displayed. The method does not require image fusion and, thus, circumvents possible localization errors associated with the acquisition and fusion of several 3D MR imaging data sets (12).
References
- 1.Wyler AR, Walker G, Richey ET, Hermann BP. Chronic subdural strip electrode recordings for difficult epileptic problems. J Epilepsy 1988;1:71–78 [Google Scholar]
- 2.Risinger MW. Electroencephalographic strategies for determining the epileptogenic zone. In: Lüders HO, ed. Epilepsy Surgery. New York, NY: Raven;1992. :337–347
- 3.Roper SN. Implantation of grid and strip electrodes. Tech Neurosurg 1995;1:5–10 [Google Scholar]
- 4.Behrens E, Zentner J, van Roost D, Hufnagel A, Elger CE, Schramm J. Subdural and depth electrodes in the presurgical evaluation of epilepsy. Acta Neurochir 1994;128:84–87 [DOI] [PubMed] [Google Scholar]
- 5.Winkler PA, Vollmar C, Krishnan KG, Pfluger T, Brückmann H, Noachtar S. Usefulness of 3-D reconstructed images of the human cerebral cortex for localization of subdural electrodes in epilepsy surgery. Epilepsy Res 2000;41:169–178 [DOI] [PubMed] [Google Scholar]
- 6.Wellmer J, Widman G, v. Oertzen J, et al. Anatomically precise and reproducible localization of grid electrodes in invasive presurgical epilepsy diagnosis: a method for routine application. Epilepsia 2000;41(suppl 7):79 [Google Scholar]
- 7.Bastos AC, Comeau RM, Andermann F, et al. Diagnosis of subtle focal dysplastic lesions: curvilinear reformatting from three-dimensional magnetic resonance imaging. Ann Neurol 1999;46:88–94 [DOI] [PubMed] [Google Scholar]
- 8.Wyllie E, Lüders H, Morris HH, et al. Subdural electrodes in the evaluation for epilepsy surgery in children and adults. Neuropediatrics 1988;19:80–86 [DOI] [PubMed] [Google Scholar]
- 9.Bastos AC, Araujo D Jr, Santos AC, et al. MRI curvilinear reformatting: a new tool to define relationship between subdural grids and cortical areas [abstract]. Epilepsia 1998;39:6 [Google Scholar]
- 10.Bootsveld K, Traber F, Kaiser WA, et al. Intracranial EcoG electrodes: location determination using three-dimensional reconstruction of MR data of the brain as a component of the presurgical diagnosis of epilepsy. Radiologe 1993;33:185–188 [PubMed] [Google Scholar]
- 11.Grzeszcuk R, Tan KK, Levon DN, et al. Retrospective fusion of radiographic and MR data for localization of subdural electrodes. J Comput Assist Tomogr 1992;16:764–773 [DOI] [PubMed] [Google Scholar]
- 12.Hill DL, Castellano Smith AD, Simmons A, et al. Sources of error in comparing functional magnetic resonance imaging and invasive electrophysiological recordings. J Neurosurg 2000;93:214–223 [DOI] [PubMed] [Google Scholar]