Abstract
Summary: We report the MR imaging findings of a solitary fibrous tumor involving the parapharyngeal space. The tumor was a well-circumscribed solid mass with a lobulated contour. It had the same signal intensity as the muscle on T1-weighted MR images, heterogeneously high signal intensity on T2-weighted images, and homogeneous strong enhancement after the administration of contrast material. It mimicked a tumor originating from the deep lobe of the parotid gland.
Solitary fibrous tumor (SFT) is an uncommon spindle cell tumor that usually occurs in the pleura. Recently, the presence of these tumors was reported in a number of extrapleural sites, including the head and neck. The parapharyngeal space is a rare location for a SFT (1). We describe the MR imaging findings in a case of a SFT that involved the parapharyngeal space.
Case Report
A 49-year-old woman was admitted with a 6-month history of a bulging mass in the left oral cavity and a mild sore throat. She did not have any difficulty swallowing or breathing. She had been treated for left otitis media at a local hospital for 1 year prior to this admission.
Oropharyngeal examination revealed a large, smooth swelling that occupied the left tonsillar fossa; left palatine tonsillar hypertrophy; and pharyngeal injection. Examination of the neck revealed a rubbery mass in the left submandibular space.
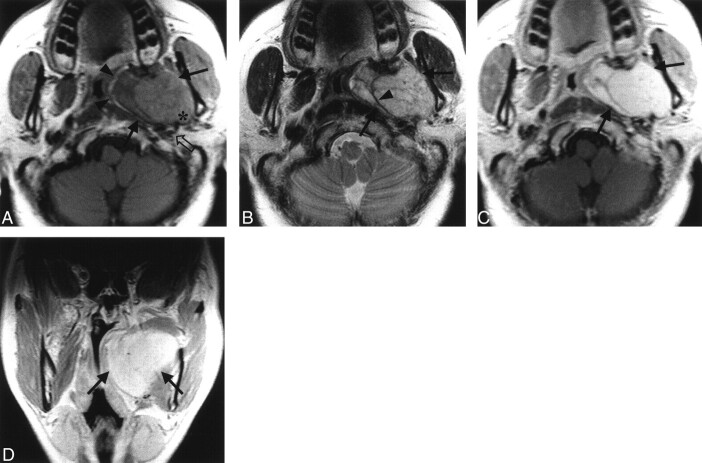
On the MR images, the mass was located in the left parapharyngeal space, which medially displaced the parapharyngeal fat and medial pterygoid muscle and posterolaterally displaced the carotid sheath. It had well-defined margin and a lobulated contour. This tumor was isointense relative to muscle on T1-weighted images (Fig 1A) and heterogeneously hyperintense relative to muscle on T2-weighted images (Fig 1B). On T1- and T2-weighted images, a linear, nonenhancing area of hypointense signal was present in an arborizing pattern within the mass, which had a hypointense rim, except for the linear area in an arborizing pattern (Fig 1B and C). The tumor was sharply demarcated from the parotid gland, and the stylomandibular tunnel was widened. The mass occupied the left parapharyngeal space at the oropharyngeal level and had homogeneously strong enhancement on the contrast-enhanced T1-weighted coronal image (Fig 1D). No cervical lymphadenopathy was detected.
Fig 1.
MR images in a 49-year-old woman with a bulging mass in the left oral cavity.
A, Axial T1-weighted (450/12) image shows a well-defined, lobulated, contoured mass in the left parapharyngeal space that has the same signal intensity as that of the adjacent muscle (solid arrows). The mass displaces the parapharyngeal fat medially (arrowheads) and the carotid sheath posterolaterally (open arrow). The mass has a clear margin against the deep lobe of the parotid gland with the widening of the stylomandibular tunnel (*).
B, Axial T2-weighted (3700/99) image obtained at the same level as in A shows the heterogeneous high signal intensity of the mass (arrows). Note the linear area of hypointense signal in an arborizing pattern within the mass (arrowhead).
C, Contrast-enhanced axial T1-weighted (450/12) image obtained at the same level as in B reveals homogeneously strong enhancement in the mass (arrows).
D, On this contrast-enhanced coronal T1-weighted (450/12) image, the mass (arrows) occupies the left parapharyngeal space at the oropharyngeal level.
Excision of the mass was performed via a transcervical approach below the mandibular angle. The mass was found near the glossopharyngeal nerve. A 5 × 4 × 4-cm, well-defined, round mass was removed en bloc with the surrounding soft tissue.
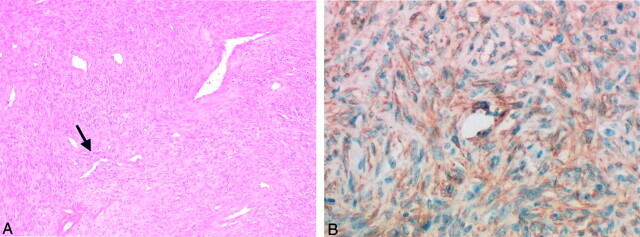
Gross pathologic examination revealed a lobulated well-encapsulated mass. The capsule of the mass was grayish white and glistening, and it contained small blood vessels. The cut surface of the mass was yellowish white, septated, and solid. Neither hemorrhage nor necrosis was present. Microscopically, the tumor had a pattern of a hemangiopericytomatous area alternating with a hypocellular fibrous region; this feature suggested a SFT (Fig 2A). With immunohistochemical staining, the neoplastic cells had a positive reaction to CD34 (Fig 2B) and negative reactions to CD31, desmin, cytokeratin, and S-100 protein.
Fig 2.
Photomicrographs in a 49-year-old woman with a bulging mass in the left oral cavity.
A, Image of the parapharyngeal mass shows that the tumor has the alternating pattern of hemangiopericytomatous area (arrow) with a hypocellular fibrous region (ie, patternless pattern); these features suggest an SFT (hematoxylin-eosin stain, original magnification ×40).
B, Image shows that the neoplastic cells have a positive reaction with CD34 immunohistochemical staining; this finding clarifies the diagnosis of SFT (original magnification ×200).
Postoperatively, the patient recovered well, without any neurologic deficits. She also received radiation therapy with 50 Gy for 6 weeks.
Discussion
SFTs most often occur on the pleura of the lung, where they are also called fibrous mesothelioma or localized fibrous tumors. These neoplasms have been described within the lung proper and in a variety of extrapleural sites, including the mediastinum, pericardium, peritoneum, retroperitoneum, orbit, and thyroid gland. Recently, the presence of SFTs has been reported in the upper aerodigestive tract, with the most common location being the nasal cavity and/or paranasal sinuses, followed by the nasopharynx, parapharyngeal space, and supraglottic larynx (1, 2). The parapharyngeal SFT can be confused with a variety of other neoplasms due to its rarity.
SFTs occur with equal frequency in both sexes, and they usually occur in adults aged 30–64 years. Clinically, SFTs typically have one or more of the following features: 1) symptoms related to the site of the tumor; 2) systemic symptoms, eg, hypoglycemia, arthralgia, osteoarthropathy, and clubbing of the fingers; and 3) incidental clinical detection or radiologic findings, such as a well-circumscribed solid mass with hypo- to isointensity on T1-weighted images, heterogeneous hyperintensity on T2-weighted images, and a variable degree of the contrast enhancement. All of these symptoms resolve with removal of the tumor (1, 2).
Symptoms related to the masses within the parapharyngeal space are often minimal; the most common symptom is a bulge in the overlying pharynx, as in this case (3).
The clinical behavior of SFTs is unpredictable. Approximately 80–88% of pleural SFTs behave in a benign fashion and are cured with surgical resection. About 12–20% of SFTs are associated with invasion, recurrence, and metastasis (4). Aggressiveness is usually associated with large tumors, hypercellularity, increased mitoses, pleomorphism, and necrosis. The most important prognostic factor, however, is the resectability of the tumor (5).
Grossly, SFTs are usually well-circumscribed, with a smooth external surface. The cut surfaces are generally pale and firm. Microscopically, SFTs are well-circumscribed but non-encapsulated tumors; they are recognized as having a characteristic patternless arrangement consisting of a varying number of spindle cells that are randomly arranged in a collagenous background of variable vascularity. Most tumors have hypercellular and hypocellular areas; often, a portion of the tumor has prominent branching vessels and a hemangiopericytoma-like pattern (4, 6).
The histologic features of SFTs may be confused with those of a hemangiopericytoma, schwannoma, fibrous histiocytoma, fibrosarcoma, or nasopharyngeal angiofibroma. In particular, the tumors can simulate hemangiopericytoma because of the presence of compact cellular foci surrounding the vascular channels (7). CD34 immunohistochemical staining has been shown to be strongly in SFTs. Negative immunoreactive results with CD31, cytokeratin, desmin, and S-100 protein may exclude the other differential diagnoses, such as hemangiopericytoma, epithelial tumor, fibrosarcoma, and neurogenic tumor. The correct diagnosis of our case was also established with the positive immunoreactivity for CD34 and negative reactivity for other markers such as CD31, cytokeratin, desmin, S-100 protein (8).
The reported MR imaging findings of SFTs include isointense signal on T1-weighted images and hypointense signal on T2-weighted images, with prominent and heterogeneous enhancement. These features correspond well to the histologic findings of a fibrous stroma intermixed with prominent vascular channels (4).
The differential diagnosis in our case included a deep lobe tumor of the parotid gland, nerve sheath tumor, paraganglioma, and retropharyngeal lymphadenopathy.
A tumor in the deep lobe of the parotid gland, such a pleomorphic adenoma, is the most common tumor in the parapharyngeal space. It usually has heterogeneous and intermediate signal intensity on T1-weighted MR images and intermediate-to-high signal intensity on T2-weighted images. Pleomorphic adenoma often has a cystic change as well. Images in our case also revealed widening of the stylomandibular tunnel due to a mass effect on the styloid process; these features mimicked those of a deep lobe tumor of the parotid gland.
Usually, schwannomas are located in the carotid space, displace the internal carotid artery anteromedially, have heterogeneous signal intensity due to cystic change and hemorrhage, and have intensely enhancement after the administration of contrast material.
Typically, paragangliomas in this area anteriorly displace the internal carotid artery because of its origin around the vagus nerve in the carotid space. This hypervascular tumor has multiple focal and serpentine areas of low signal intensity that represent vascular flow voids within the mass, especially in large tumors (9).
Large retropharyngeal lymphadenopathy, which is observed in Castleman disease, appears as a well-marginated, homogeneously enhancing mass. Nodes are isointense and hyperintense on T1- and T2-weighted images, respectively. They displace the parapharyngeal fat anteriorly, the styloid process anterolaterally, and the carotid space laterally (9).
Conclusion
In conclusion, SFT is a rare mass that may involve the parapharyngeal space. On MR images, it is a well-defined, lobulated, contoured mass. It is isointense relative to muscle on T1-weighted images and heterogeneously hyperintense on T2-weighted images, with strong and heterogeneous enhancement. SFT should be considered in the differential diagnosis of masses involving the parapharyngeal space.
References
- 1.Gangopadhyay K, Taibah K, Manohar MB, Kfoury H. Solitary fibrous tumor of the parapharyngeal space: A case report and review of the literature. Ear Nose Throat J 1996;75:681–684 [PubMed] [Google Scholar]
- 2.Ferreiro JA, Nascimento AG. Solitary fibrous tumour of the major salivary glands. Histopathology 1996;28:261–264 [DOI] [PubMed] [Google Scholar]
- 3.Allison R, Van Der Waal I, Snow G. Parapharyngeal tumors: a review of 23 cases. Clin Otolaryngol 1989;14:199–203 [DOI] [PubMed] [Google Scholar]
- 4.Kim TA, Brunberg JA, Pearson JP, Ross DA. Solitary fibrous tumor of the paranasal sinuses: CT and MR appearance. AJNR Am J Neuroradiol 1996;17:1767–1772 [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler A, Lapinsky J, Berenholz L, Sarfaty S, Segal A. Solitary fibrous tumor of the nasal cavity. Otolaryngo1 Head Neck Surg 1999;121:826–828 [DOI] [PubMed] [Google Scholar]
- 6.Zukerberg LR, Rosenberg AE, Randolph G, Pilch BZ, Goodman ML. Solitary fibrous tumor of the nasal cavity and paranasal sinuses. Am J Surg Pathol 1991;15:126–130 [DOI] [PubMed] [Google Scholar]
- 7.Kohmura T, Nakashima T, Hasegawa Y, Matsuura H. Solitary fibrous tumor of the paranasal sinuses. Eur Arch Otorhinolaryngol 1999;256:233–236 [DOI] [PubMed] [Google Scholar]
- 8.Rosai J. Ackerman’s Surgical Pathology. St Louis, Mo: Mosby;1996. :34–45
- 9.Shin JH, Lee HK, Kim SY, et al. Castleman’s disease in the retropharyngeal space: CT and MR imaging findings. AJNR Am J Neuroradiol 2000;21:1337–1339 [PMC free article] [PubMed] [Google Scholar]