Abstract
Summary: We report a case of atypical cerebral venous drainage in a 38-year-old woman with symptoms of benign paroxysmal positional vertigo. Thrombosis of the left internal jugular vein and sigmoid sinus was suspected on the basis of spin-echo and time-of-flight MR findings, but multisection CT angiograms showed a patent sigmoid sinus and predominant drainage via the emissary veins toward the vertebral plexus, with only a minor contribution of the jugular veins. This case illustrates the variability of the venous anatomy in the craniocervical region.
Internal jugular vein (IJV) and sigmoid sinus thrombosis was suspected because of the following: 1) absence of flow-induced signal alterations on MR images, 2) apparent collateral flow through strongly dilated intraspinal and vertebral veins, and 3) low bidirectional flow on color-coded duplex images of the IJV. However, contrast-enhanced MR venography and multisection CT angiography (CTA) demonstrated the patency of the vessel, but the vessel had only a minor contribution to the cerebrovenous drainage, which was largely dependent on the vertebral venous system. This case report underlines the high degree of variability of the venous anatomy in the craniocervical region.
Case Report
A 38-year-old woman had symptoms that were highly suggestive of benign paroxysmal positional vertigo. However, clinical examination findings, including those with repeated positional maneuvers, remained normal. Because the patient also had dizziness and a feeling of intracranial pressure in the supine position, her neurologist ordered an outpatient cranial MR imaging examination to exclude underlying brain disease. Because a high-flow signal void was absent in the left jugular vein on T1- and T2-weighted spin-echo MR images obtained at the level of the skull base and lower, MR venography (MRV) was performed by using the two-dimensional (2D) time-of-flight (TOF) technique. On the images, the proximal IJV and sigmoid sinus were not depicted on the left side (Fig 1A and B). Furthermore, intraspinal veins, as well as the venous vessels surrounding the cranial portion of the vertebral arteries and the segmental interconnections of the two systems, appeared remarkably prominent (Fig 1C and D). This finding led the radiologist to suspect thrombosis of the left IJV and sigmoid sinus, with collateral drainage through these secondary systems. No dilated cortical veins or parenchymal lesions were evident.
Fig 1.
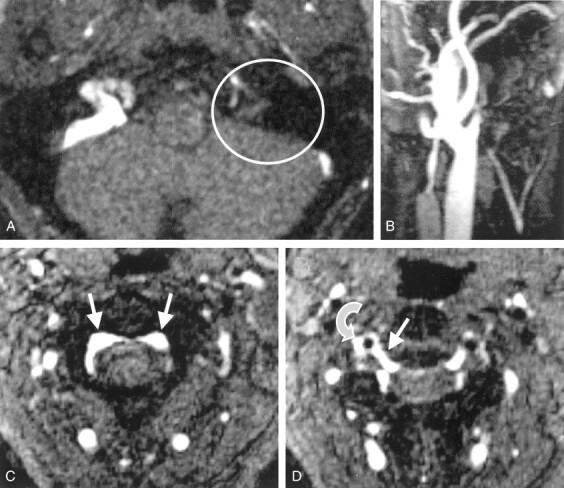
Cervical 2D TOF MR angiograms.
A, Axial source image depicts no contrast enhancement within the left sinus sigmoideus (circle).
B, Maximum intensity projection (MIP) reconstruction image of the left IJV depicts only a short proximal vessel segment with regular contrast enhancement.
C, Axial source image obtained at the level of C2 shows prominent epidural veins of the anterior intraspinal system (arrows).
D, Axial source image obtained at the level of the C2–3 intervertebral space shows that a radicular vein (straight arrow) connects the anterior intraspinal system to the right vertebral vein (curved arrow) next to the vertebral artery (flow void).
The patient was admitted to our neurology department. Because the link between the clinical history and MR imaging findings appeared to be merely coincidental, we hesitated to start anticoagulation. Moreover, the imaging techniques applied (spin-echo and TOF MRV) were not suitable for definite diagnosis; thus, other diagnostic modalities were required. At duplex sonography of the cervical portion of the IJV, longitudinal and cross-sectional images showed no signs of thrombosis, and the vessel was fully compressible. However, the vein appeared small, and flow velocities were low, with a bidirectional pattern that depended on the heart cycle. The right IJV also had a small caliber and low flow velocities. Similar to the MRV results, and as an unusual duplex finding, intraspinal flow signals and segmental venous inflows to the vertebral veins were clearly discernible (Fig 2). From these findings, the left IJV appeared to make little or no contribution to the cerebral venous drainage, which was instead largely dependent on the vertebral venous system. Although no direct evidence of a thrombus was found, thrombosis of the cranial jugular vein, which was inaccessible for duplex scanning, could not be entirely ruled out. Thus, contrast-enhanced MR studies were used. The T1-weighted gradient-echo MRV image showed regular contrast enhancement within the cervical segments of the left IJV (Fig 3), but assessment of the skull base remained difficult. Consequently, multisection CTA was performed (Fig 4A). With this technique, the sigmoid sinus was definitely shown to be patent on transverse sections and multiplanar reconstructions, with hypoplasia of the sinus outflow. Moreover, multisection CTA provided detailed anatomic information about the venous outflow by depicting a prominent bilateral connection of the sigmoid sinus to the external vertebral venous plexus via the posterior condylar emissaries (Fig 4B). While these results excluded thrombosis of the sigmoid sinus or IJV, the suspected diagnosis of benign paroxysmal positional vertigo was later confirmed with the results of a positional maneuver performed immediately after the patient rested that night.
Fig 2.
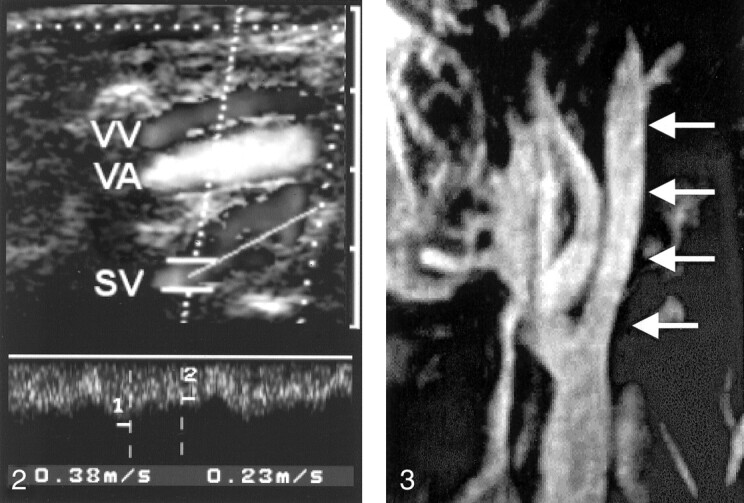
Duplex sonogram depicts the right vertebral vein (VV) and vertebral artery (VA) at the level of C4 and C5. Note the prominent segmental venous inflow (SV) with a high systolic flow velocity of 38 cm/s.
Fig 3.

MIP of the contrast-enhanced T1-weighted gradient-echo MRV image of the left IJV. The vein has good contrast enhancement below the skull base (arrows).
Fig 4.
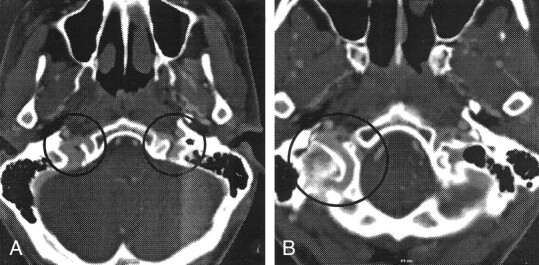
Axial images from multisection CTA.
A, Bilateral asymmetrical venous contrast enhancement at the level of the jugular foramen is depicted (circles).
B, Prominent posterior condylar emissary vein is depicted on the right side (circle).
Discussion
Thrombosis of the IJV, similar to deep venous thrombosis of the legs, is associated with a substantial risk of pulmonary embolism and requires systemic anticoagulation during the acute stage. Even more so, in dural sinus thrombosis, patient outcome and survival has been shown to depend on rigorous anticoagulation to prevent appositional growth of the thrombus and spread to cortical veins (1). However, because anticoagulation is a potentially hazardous treatment, the diagnosis needs to be accurate. While patients typically present with severe headache; seizures; and, often, focal neurologic signs (2), dural sinus thrombosis is a variable condition, and mild and subclinical courses without treatment for several months have been reported.
In the present case, thrombosis of the left IJV and sigmoid sinus was suspected on the basis of spin-echo and TOF MR findings. MR angiography is a well-established noninvasive imaging method for the examination of both intracranial and extracranial vessels, including the cerebral veins and sinus (3). However, when MRV is performed in the TOF mode, it has a tendency to exaggerate pathologic findings or produce false-positive results in situations of low or in-plane flow (3, 4). On the other hand, depiction of venous collateral flow has been described as an indirect diagnostic parameter in patients with cerebral venous thrombosis (2); this finding lent credibility to the diagnosis in our patient.
Single-section CTA is a powerful tool for assessing the venous cerebrovascular system, and CTA may be superior to MRV in depicting intracranial venous structures with low flow or small diameters (5), because the technique is not susceptible to flow-related artefacts. The recent introduction of multisection technology has substantially increased the imaging speed and spatial resolution; this permits high-resolution visualisation of longer vascular segments. In our patient, multisection CTA findings allowed us to clearly rule out thrombosis of the sigmoid sinus, and they demonstrated the presence of an anatomic variant of the venous drainage at the craniocervical junction.
Venous outflow from the superior sagittal sinus and deep cerebral veins is usually directed via the confluens sinuum toward the sigmoid sinuses and jugular veins. Interconnections with other basal venous structures permit additional drainage toward the vertebral venous system. This freely communicating, valveless system is present throughout the entire spinal column and may be divided into an internal intraspinal part, the epidural veins, and an extraspinal paravertebral part. At the craniocervical level, anterior sources of the intraspinal system arise from the basal plexus and the inferior petrosal sinus. A posterior communication exists with the occipital sinus, which may be relevant in cases of lateral sinus hypoplasia. The extraspinal system receives blood anteriorly from the cavernous sinus via the pterygoid plexus. A dorsal part is connected to the suboccipital venous plexus, which receives blood through the mastoid and condylar emissaries (6, 7). Although the jugular veins were previously thought to be the predominant draining pathway (8), recent study findings have demonstrated that this role is confined to the supine position; redirection of venous flow to the vertebral veins occurs in the upright position (9). Rather than being a minor plexus that surrounds the vertebral artery, the vertebral veins in the cervical region represent large collecting vessels of the vertebral venous system; they are readily detectable on color-coded duplex sonograms (10). They receive blood from condylar veins, from emissaries, and through segmental connections (8, 11).
Because of their ontogenetic derivation from a plexiform structure, numerous variations of the dural sinuses exist at the torcular region (12, 13). While right-sided dominance of the sigmoid sinus is common, unilateral aplasia of a sigmoid sinus occurs in 4% of cases (13), and rudimentary lateral sinuses on both sides were reported in one of 163 subjects examined with retrograde jugular venography (12). Predominant drainage via the emissary veins toward the vertebral plexus with only a minor contribution of both jugular veins, as demonstrated with multisection CTA in our patient, must be regarded as an extremely unusual finding.
Although a causal relationship between this anatomical variant and the final diagnosis of benign paroxysmal positional vertigo can definitely be ruled out, hypoplasia of the jugular venous drainage might explain the patient’s complaint of dizziness and intracranial pressure in the supine position, because the relative contribution of the IJV should be greatest under these circumstances (9). In this sense, the patient’s situation might be compared to that of patients after bilateral neck dissection with the removal of both IJVs; these patients often cannot tolerate the supine position (14).
Conclusion
Our case report reflects the well-known limitations of spin-echo and TOF MR imaging in the diagnosis of cerebrovenous disorders. More important, the need to consider the large anatomic variability of the cerebrovenous drainage, especially in the torcular region, is highlighted. Our experience confirms the excellent suitability and robustness of multisection CTA for the investigation of intracranial and cervical venous structures, even when regions of variable and complex vascular anatomy must be assessed.
Acknowledgments
The authors wish to thank Dr B. Sander for providing outpatient MR data.
References
- 1.Einhäupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet 1991;338:597–600 [DOI] [PubMed] [Google Scholar]
- 2.Einhäupl K, Masuhr F. Cerebral venous and sinus thrombosis: an update. Eur J Neurol 1994;1:109–126 [DOI] [PubMed] [Google Scholar]
- 3.Bianchi D, Maeder P, Bogousslavsky J, Schnyder P, Meuli R. Diagnosis of cerebral venous thrombosis with routine magnetic resonance: an update. Eur Neurol 1998;40:179–190 [DOI] [PubMed] [Google Scholar]
- 4.Urchuk SN, Plewes DB. Mechanisms of flow-induced signal loss in MR angiography. J Magn Reson Imaging 1992;2:453–462 [DOI] [PubMed] [Google Scholar]
- 5.Ozsvath RR, Casey SO, Lustrin ES, Alberico RA, Hassankhani A, Patel M. Cerebral venography: comparison of CT and MR projection venography. AJR Am J Roentgenol 1997;169:1699–1707 [DOI] [PubMed] [Google Scholar]
- 6.Andeweg J. The anatomy of collateral venous flow from the brain and its value in aetiological interpretation of intracranial pathology. Neuroradiology 1996;38:621–628 [DOI] [PubMed] [Google Scholar]
- 7.Eckenhoff JE. The physiologic significance of the vertebral plexus. Surg Gynec Obstet 1970;131:72–78 [PubMed] [Google Scholar]
- 8.Braun JP, Tournade A. Venous drainage in the craniocervical region. Neuroradiology 1977;13:155–158 [DOI] [PubMed] [Google Scholar]
- 9.Valdueza JM, von Münster T, Hoffmann O, Schreiber S, Einhäupl KM. Postural dependency of the cerebral venous outflow. Lancet 2000;355:200–201 [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann O, Weih M, von Münster T, Schreiber S, Einhäupl KM, Valdueza JM. Blood flow velocities in the vertebral veins of healthy subjects: a duplex sonographic study. J Neuroimaging 1999;9:198–200 [DOI] [PubMed] [Google Scholar]
- 11.Théron J, Djindjian R. Cervical phlebography using catheterization. Neuroradiology 1973;108:325–331 [DOI] [PubMed] [Google Scholar]
- 12.Dora F, Zileli T. Common variations of the lateral and occipital sinuses at the confluens sinuum. Neuroradiology 1980;20:23–27 [DOI] [PubMed] [Google Scholar]
- 13.Lang J. Skull base and related structures. In: Atlas of Clinical Anatomy. Stuttgart, Germany: Schattauer Verlag; 1995
- 14.Gius JA, Grier DH. Venous adaptation following bilateral radical neck dissection with excision of the jugular vein. Surgery 1950;28:305–321 [PubMed] [Google Scholar]


