Abstract
BACKGROUND AND PURPOSE: CT and MR imaging are useful for evaluating the extension of carcinomas in the face and neck. We evaluated the involvement by carcinoma arising from the gingiva (ie, gingival cancer) by using CT and MR imaging.
METHODS: We retrospectively examined 122 patients with squamous cell carcinoma (SCCA) in the lower (88 patients) and upper (34 patients) gingiva. Extension of SCCA into the spaces of the face and neck was evaluated with CT and MR imaging, and findings were surgically confirmed.
RESULTS: Spread into the face and neck spaces occurred in 58% of patients. The buccal space was the most common site of spread, occurring in 42% of the lower and of 47% of the upper gingival cancers. Spread into the masticator space occurred from the lower gingival cancers in the molar region (20%) but not from the anterior region. Masticator space involvement from the upper gingiva was rare (4%). The retromolar triangle and buccal space immediately anterior to the ramus served as a corridor for cancer extension from the lower gingiva into the masticator space. The sublingual space (11%) was a less common site of spread from the lower gingiva.
CONCLUSION: Gingival cancers spread into the masticator, buccal, and sublingual spaces depending on the primary sites in the oral cavity. An understanding of the face and neck-space anatomy is important in diagnosing cancer extension in the oral cavity gingiva and in treating patients with such disease.
Squamous cell carcinoma (SCCA) of the gingiva accounts for fewer than 10% of all oral cavity cancers (1). However, the extent of tumor invasion into the mandible and maxilla and further extension into the adjacent face and neck spaces are always causes for special concern because, in addition to the tumor size (2), tumor spread into such anatomic structures may lead to a poor prognosis (3). Involvement of the pterygopalatine fossa and masticator space may enable invasion along the neurovascular bundles into the cavernous sinus via the maxillary and mandibular nerves (1). Thus, knowledge of the extent of gingival cancer is important for staging the cancer before surgery or radiation therapy.
CT and MR imaging are useful in evaluating the extent of oral cancers (1, 4–7). Mucosal spread can be easily assessed with physical examination, but deep spread cannot; this is where imaging has a role. To our knowledge, no extensive study of CT and MR imaging has been performed to assess tumor extension from SCCA of the gingiva. The purpose of our study was to evaluate the involvement by carcinoma arising from the gingiva (ie, gingival cancer) by using CT and MR imaging. Herein, we report the various pathways of primary tumor spread from upper and lower gingival cancers. We studied both the normal anatomy and observed patterns of gingival cancer spread.
Methods
Patients
The study population comprised 122 patients with upper gingival SCCA (n = 34; 19 men and 15 women; age range, 45–87 years; average age, 69 years) or lower (n = 88; 40 men and 48 women; age range, 48–91 years; average age, 68 years) gingival SCCA. Each of the two patient groups was further divided into two subgroups: patients with gingival cancer in the anterior region (11 upper and 28 lower gingival cancers) and those with gingival cancer in the molar region (23 upper and 60 lower gingival cancers). Gingival cancers in the anterior region were those located within the region from the incisor to the premolar. Gingival cancers in the molar region were those located in the molar region, retromolar region, or both. The present study did not include patients who had gingival cancer that spanned the anterior and molar regions. The extent of tumor invasion into the surrounding structures of the alveolar ridge, mandible, maxillary sinus, and facial and neck spaces was confirmed at surgery. In the upper gingiva, carcinomas arose from the buccal aspect (n = 9), palatal aspect (n = 14), or both (n = 11). In the lower gingiva, carcinomas arose from the buccal aspect (n = 34), lingual aspect (n = 30), and both (n = 24).
The image file at our hospital contained two additional cases in which cancers occupied both the anterior and molar regions of the gingiva. Images in one of them, that of a 76-year-old woman, showed that the cancer was confined in the gingiva without deep extension. Images in the other case, that of a 60-year-old woman, showed that cancer deeply extended into the alveolar bone, causing destruction of the buccal cortex, and further into the buccal fat pad. We excluded these patients from the present assessment.
CT Imaging
All 122 patients underwent imaging with the use of a HiSpeed Advantage SG CT imaging system (GE Medical Systems, Milwaukee, WI). The scanning orientation was parallel to the Frankfurt horizontal line or inferior border of the mandible. The patients were scanned from the top or level of the orbita to the level of the clavicle. Scanning was performed just after an intravenous bolus injection of approximately 100 mL (2 mL/kg) of iopamidol (Iopamiron 300; Schering, Berlin, Germany) at a rate of 1.0 mL/s. Scanning required about 2–3 minutes. In some patients, imaging was performed as just described, and then we scanned the maxilla and mandible with the scanning orientation parallel to the inferior border of the mandible, avoiding artifacts from dental fillings as much as possible. In these cases, scanning required 3–4 minutes. When the contrast medium was injected at a rate of 1 mL/s, sufficient enhancement were sustained in the parotid and submandibular glands and in the masseter muscle for at least 8 minutes after the injection was completed. Therefore, we believe that the contrast enhancement effect in the organs in the face and neck region was not substantially delayed. Scanning was performed with a collimation of 3 mm, pitch of 1:1, 512 × 512 matrix, display field of view of 23 cm, 120 kVp, and 200 mA.
We assessed, by using CT images, the extension pathways from gingival cancers and determined which of the facial and neck spaces (including the masticator, parotid, parapharyngeal, retropharyngeal, buccal, submandibular, and sublingual spaces) had tumoral involvement. Reconstructed coronal images were obtained to assess cancer spread patterns in some patients. In all patients, we reviewed both bone and soft tissue windows.
MR Imaging
MR imaging was performed in 41 patients with upper (n = 13) and/or lower (n = 28) gingival cancer. Patients were examined with a 1.5-T MR imaging system (Horizon LX CV/NV; GE Medical Systems) or a 1.0-T MR imaging system (Expert; Siemens Medical Systems, Erlangen, Germany). A head coil was used for imaging. Axial and coronal T1-weighted images (TR/TE/NEX, 466/14/2 or 500/14/2) were obtained by using a conventional spin-echo sequence with or without intravenous contrast enhancement. We also obtained axial and coronal fat-saturated T2-weighted images (3000/106/2 or 3200/96/2) by using a fast spin-echo sequence. Fat-saturated T1-weighted imaging was performed after gadolinium enhancement with gadopentetate meglumine (Magnevist; Schering, Berlin, Germany) in 14 patients (five patients with upper gingival cancer and nine patients with lower gingival cancer). The section thickness was 4 mm. MR imaging was performed with a 256 × 224 matrix and an intersection gap of 1.0 mm.
Results
Correlation between Physical and Imaging Findings
All seven patients with trismus had imaging findings that were suggestive of cancer spread into the masticator space, and all 18 patients with cheek swelling had imaging findings that were suggestive of cancer spread into the buccal space. On the other hand, six of 13 patients with imaging findings suggestive of cancer spread into the masticator space did not have trismus, and 35 patients with imaging findings suggestive of cancer spread into the buccal space did not have cheek swelling.
Lower Gingival Cancer
The profiles of cancer spread from lower and upper gingival cancers are summarized in the Table. Lower gingival cancers destroyed the alveolar bone in 75 (85%) of 88 patients. Lower gingival cancers, with or without alveolar bone destruction, spread into the face and neck spaces in 51 (58%) of the 88 patients.
Profiles of gingival cancer spread into the face and neck spaces
| Space or Structure | Lower Gingiva |
Upper Gingiva |
Total(n = 122) | ||
|---|---|---|---|---|---|
| Anterior(n = 28) | Molar(n = 60) | Anterior(n = 11) | Molar(n = 23) | ||
| Sublingual space | 1 | 9 | 0 | 0 | 10 |
| Buccal space | 4 | 33 | 7 | 9 | 53 |
| Masticator space | 0 | 12 | 0 | 1 | 13 |
| Mentum | 3 | 0 | 0 | 0 | 3 |
| Maxillary sinus | 0 | 0 | 2 | 8 | 10 |
| Nasal cavity | 0 | 0 | 2 | 0 | 2 |
The buccal space was most commonly involved (42%) in 88 patients with lower gingival cancer, and cancers in the molar region (33 [89%] of 37 patients with buccal space involvement from lower gingival cancer) contributed to this (Fig 1). Lower gingival cancers from the molar region spread into the masticator space in 12 (20%) of 60 patients, but those from the anterior region did not (0 of 28 patients). Eight of 12 patients with lower gingival cancer spread in the masticator space had simultaneous involvement of the buccal spaces. Of these, five patients had extensive bone destruction in the anterior mandibular ramus. The remaining four patients with masticator space involvement had cancers that spread into the fat space between the medial pterygoid muscle and ramus but not bone destruction of the ramus. All of these patients also had enlarged and irregularly enhancing medial pterygoid muscles. One patient had cancer that had entered the infratemporal and temporal portions of the masticator space (Fig 2) and then the skull base.
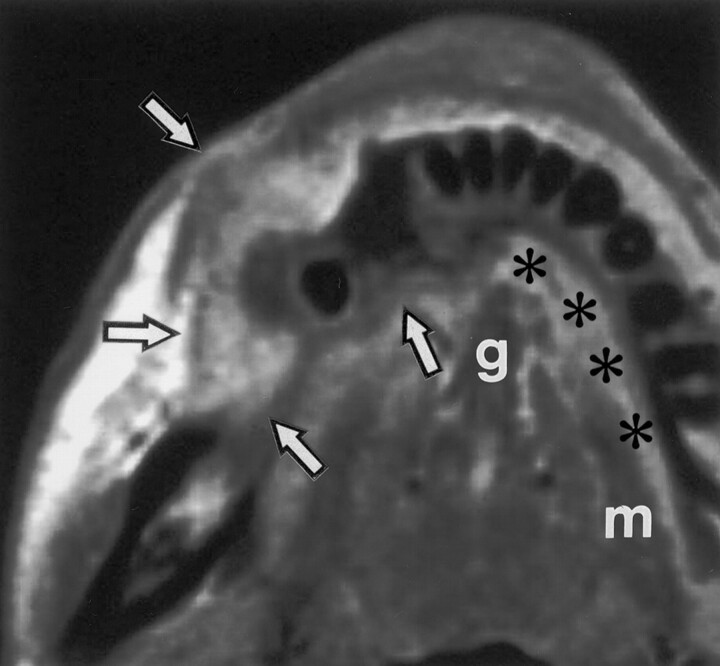
Fig 1.
Axial T1-weighted MR image in a 69-year-old woman with lower gingival cancer in the right molar region shows cancer (arrows), which is spreading into the buccal and sublingual spaces (*). The sublingual space is superomedial to the mylohyoid muscle (m). The genioglossus muscle (g) borders the space medially, and the lingual aspect of the mandible borders it anteriorly and laterally. The tumor spreads posteriorly into the retromolar trigone and contiguously from the buccal space.
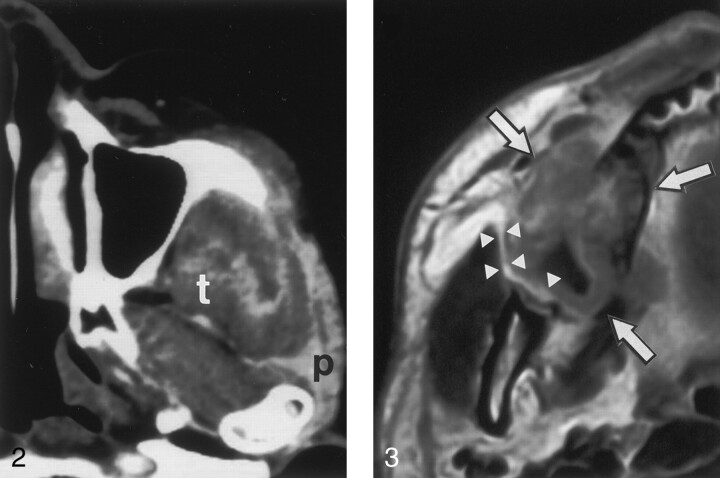
Fig 2.
Axial CT scan in a 72-year-old woman with lower gingival cancer in the left molar region shows that cancer involves the infratemporal and temporal portions of masticator space. The tumor extends into the parotid gland (p) and the deep belly of the temporalis muscle (t).
The sublingual space was the third most common site of invasion from lower gingival cancer (Fig 1), and it was more frequently involved by gingival cancers from the molar region (15%) than by those from the anterior region (4%). No further spread from the sublingual space into the submandibular space was observed. Some lower gingival cancers in the anterior region extended from the alveolar bone to the mentum (11%).
Involvement of the buccal, masticator, or sublingual spaces occurred without any apparent alveolar ridge destruction in six patients with lower gingival cancer.
Upper Gingival Cancer
Thirty (88%) of 34 patients with upper gingival cancer had alveolar bone destruction. The buccal space was involved in 16 (47%) of 34 patients (Fig 3). Cancer more frequently spread into the buccal space from upper gingival cancers in the anterior region (64%) than from those in the molar region (39%). Face and neck space involvement by upper gingival cancer was not observed in patients who did not have cancerous alveolar ridge destruction.
Fig 3.
Axial gadolinium-enhanced T1-weighted MR image in a 45-year-old man with upper gingival cancer in the right molar region shows cancer (arrows), which is spreading into the buccal space but not the masticator space. Note that a thin fat layer (arrowheads) is present between the cancer and mandibular ramus.
Upper gingival cancer spread into the masticator space in only a single patient in whom the extending cancer destroyed the posterior part of the maxilla. This finding implied that the observed uncommon involvement of the masticator space by the upper gingival cancer was a result of the cancer spreading into the pterygopalatine fossa via the maxillary sinus.
The maxillary sinus was more frequently invaded by upper gingival cancers from the molar region (35%) than by those from the anterior region (18%). In two patients, cancers from the anterior region spread into the nasal cavity.
Discussion
Our findings showed that the buccal space was the most common site of spread from upper and lower gingival cancers. The masticator space was the second most common site of spread of cancer arising from the lower gingiva in the molar region. Gingival cancers did not spread into the masticator space from other sites (ie, upper gingival cancers and lower gingival cancers of the anterior region), except in a single patient in whom the masticator space was involved because of secondary extension from the maxillary sinus.
The spectrum of lesions that involve the buccal space includes minor salivary gland tumors, hemangiomas, and soft tissue sarcomas (8), as well as SCCA of the gingiva. The buccal space is often simultaneously involved when infection or malignancy is present in the masticator space (9). In our study, eight patients with lower gingival cancer had simultaneous involvement of the masticator and buccal spaces. Cancers spread into the face and neck spaces chiefly by means of direct extension from the primary site (1). However, how cancer in the molar region spreads into the masticator space is unclear. In the mandible, the buccinator muscle originates from the buccinator crista, which course along the anteromedial aspect of the ramus and then in the retromolar region of the alveolar ridge (Fig 4). Because the muscle fascia continues to the periosteum at its attachment (10–12), direct invasion into the masticator space from the molar region requires the tumor to bypass this muscle insertion point.
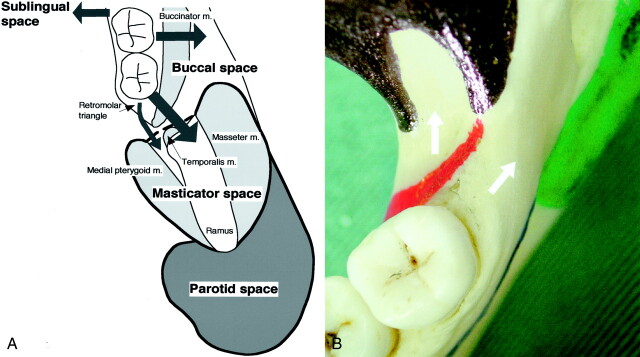
Fig 4.
Extension pathways of lower gingival cancer.
A, Diagram shows the pathways for the spread of lower gingival cancers in molar region into the buccal, masticator, and sublingual spaces. The retromolar triangle or buccal space immediately anterior to mandibular ramus serves as a corridor for cancers to spread into the masticator space. Note that a fat space exists between the medial pterygoid muscle and mandibular ramus; this is a site at which cancer can spread from the retromolar triangle without substantial bone destruction. In contrast, cancers that spread into the buccal space, after tunnelling through the alveolar ridge and then bypassing the buccinator muscle attachment, may destroy the anterior portion of mandibular ramus and invade the masticator space.
B, Photograph shows the topological relationship of lower gingival cancers that spread into masticator space and the muscle attachments to mandible. Colored areas indicate muscle attachments to the mandible: red indicates the buccinator; black, temporalis; green, medial pterygoid; and blue, mylohyoid. Arrows indicate two pathways for lower gingival cancer spread into the masticator space.
A potential pathway of gingival carcinoma spread into the masticator space is via the buccinator muscle, which may be involved by tumor that has tunnelled into the alveolar bone. The buccal fat pad is contiguous with the anterior aspect of the mandibular ramus (Fig 4A). Therefore, cancer that extends into the posterior portion of the buccal space immediately anterior to the mandibular ramus may invade the mandibular ramus and masticator space relatively easily (Fig 4).
An alternative pathway of spread may be via the retromolar triangle and then into the fat space between the medial pterygoid muscle and mandibular ramus (Fig 4). This pathway does not require any extensive destruction of the alveolar ridge to bypass the buccinator muscle. Indeed, in the present study, none of four cancers that spread into this fat space was associated with apparent bone destruction of the alveolar ridge or ramus. Collectively, the buccal space immediately anterior to the mandibular ramus and retromolar triangle serve as a corridor for the spread of gingival cancers into the masticator space.
Once gingival cancer spreads into the masticator space, it may progress further into the temporal space or skull base, as it did in one of our patients (Fig 2). The temporal space is superficial and accessible for clinical assessment. However, tumor invasion in the skull base is more readily delineated by using CT or MR imaging (1, 13). The masticator space contains the mandibular division of the trigeminal nerve, which enters into skull via the foramen ovale (14). Some types of cancer, such as adenoid cystic carcinoma, can extend by means of perineural tumor spread along this nerve into the cranial cavity (1). A single case was reported to have possible perineural spread of lower gingival cancer into the middle cranial fossa (15). However, in the present retrospective study population, we did not find any apparent evidence of cranial invasion. Careful examination with MR imaging is required for this end.
The sublingual space was the third most common site for tumor spread from lower gingival cancer. This space was also a common site for cancers spreading from the tongue. A previous study (4) showed that 44% of gingival carcinomas and 56% of tongue carcinomas had invaded the sublingual space. The incidence of tumor spread from the gingiva into this space was be notably less (11%). This discrepancy may be partly due to the superiority of MR imaging in depicting tumors that invade the oral floor (4), although CT has a relatively high sensitivity and specificity in the detection of cancer invasion into that space (16).
The mylohyoid muscle is occasionally defective in its anterior portion and has a slit in its posterior portion. In addition, the sublingual space continues posteriorly to the submandibular space beyond the posterior border of this muscle. This spatial relationship allows us to expect that cancers invading into the sublingual space may further spread into the submandibular space. However, we did not find any apparent involvement of the submandibular space in the present study population.
Conclusion
We conclude that the buccal space is a critical site for gingival cancer spread. Therefore, the space should be monitored carefully by using CT and MR imaging for possible cancer extension, because involvement of the posterior part of the buccal space may lead to a further extension of the cancer into the masticator space and then into the skull base. Masticator space involvement by lower gingival cancers may occur in the presence or absence of alveolar ridge destruction. In either case, the invading cancers reach the masticator space via the retromolar corridor. Extension of the upper gingival cancer into the masticator space is relatively rare, but it can occur via a different route with lower gingival cancers in cases of maxillary sinus involvement.
References
- 1.Smoker WRK. Oral cavity. In: Som PM, Curtin HD, eds. Head and Neck Imaging. 3rd ed. St Louis, Mo: Mosby;1996. :488–544
- 2.Overholt SM, Eicher SA, Wolf P, Weber RS. Prognostic factors affecting outcome in lower gingival carcinoma. Laryngoscope 1996;106:1335–1339 [DOI] [PubMed] [Google Scholar]
- 3.Hirai T, Korogi Y, Hamatake S, et al. Stages III and IV squamous cell carcinoma of the mouth: three-year experience with superselective intraarterial chemotherapy using cisplatin prior to definitive treatment. Cardiovasc Intervent Radiol 1999;22:201–205 [DOI] [PubMed] [Google Scholar]
- 4.Sumi M, Izumi M, Yonetsu K, Nakamura T. Sublingual gland: MR features of normal and diseased states. AJR Am J Roentgenol 1999;172:717–722 [DOI] [PubMed] [Google Scholar]
- 5.Yasumoto M, Shibuya H, Takeda M, Korenaga T. Squamous cell carcinoma of the oral cavity: MR findings and value of T1-versus T2-weighted fast spin-echo images. AJR Am J Roentgenol 1995;164:981–987 [DOI] [PubMed] [Google Scholar]
- 6.Yasumoto M, Shibuya H, Fukuda H, Takeda M, Mukai T, Korenaga T. Malignant lymphoma of the gingiva: MR evaluation. AJNR Am J Neuroradiol 1998;19:723–727 [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshioka H, Kamada T, Kandatsu S, et al. MRI of mucosal malignant melanoma of the head and neck. J Comput Assist Tomogr 1998;22:492–497 [DOI] [PubMed] [Google Scholar]
- 8.Tart RP, Kotzur IM, Mancuso AA, Glantz MS, Mukherji SK. CT and MR imaging of the buccal space masses. Radiographics 1995;15:531–550 [DOI] [PubMed] [Google Scholar]
- 9.Harnsberger HR. Handbook of Head and Neck Imaging. 2nd ed. St Louis, Mo: Mosby;1995. :46–59
- 10.Rudd RW, Tebo HG, Pinero GJ. Utilization of cadaver tissue for a scanning electron microscopic study of the insertion of the masseter muscle. J Prosthet Dent 1979;41:331–339 [DOI] [PubMed] [Google Scholar]
- 11.Hwang K, Kim DJ. Attachment of the deep temporal fascia to the zygomatic arch: an anatomic study. J Craniofac Surg 1999;10:342–345 [DOI] [PubMed] [Google Scholar]
- 12.Herms T, Tillmann B. Tendon entheses of the human masticatory muscles. Anat Embryol 2000;202:201–208 [DOI] [PubMed] [Google Scholar]
- 13.Chong VF, Fan YF. Pictorial review: radiology of the masticator space. Clin Radiol 1996;51:457–465 [DOI] [PubMed] [Google Scholar]
- 14.Kamel HAM, Toland J. Trigeminal nerve anatomy: illustrated using examples of abnormalities. AJR Am J Roentgenol 2001;176:247–251 [DOI] [PubMed] [Google Scholar]
- 15.Fujita M, Hirokawa Y, Naito K, Tagasahira N, Yajin K, Wada T. Recurrent lower gingival squamous cell carcinoma spreading along the pathways of the inferior alveolar nerve. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;80:369–375 [DOI] [PubMed] [Google Scholar]
- 16.Mukherji SK, Weeks SM, Castillo M, Yankaskas BC, Krishnan LAG, Schiro S. Squamous cell carcinomas that arise in the oral cavity and tongue base: can CT help predict perineural or vascular invasion? Radiology 1996;198:157–162 [DOI] [PubMed] [Google Scholar]