Abstract
Summary: Sturge-Weber syndrome was diagnosed in a neonate on basis of a characteristic port-wine stain. In the absence of any acute neurologic episode, MR images obtained when the infant was aged 3 months showed a typical pial vascular dysplasia, as well as prominent hypotrophy of the homolateral hemisphere. Areas suggesting the presence of developmental dysplasia of the cerebral mantel were found in association with the typical pial vascular anomaly. The prenatal effect of Sturge-Weber disease on normal brain development may best be explored by using a better evaluation with cerebral imaging shortly after birth.
Sturge-Weber disease (SWD) is characterized by a congenital nonevolutive port-wine facial stain found in association with a leptomeningeal microvenular dysplasia involving the ipsilateral cerebral hemisphere. The latter is frequently responsible for epileptic and hemiplegic complications that occur during the child’s first or second year of life (1). Cerebral imaging findings are often reported as being normal in the neonatal period. The leptomeningeal vascular dysplasia progressively becomes more apparent, and ipsilateral hemipheric atrophy slowly develops as a secondary feature. The hemiatrophy is thought to be causally related to both venous stasis from the pial malformation and repetitive episodes of hemiconvulsions (2). In contrast with the usual presentation, that of the present case suggests that the cerebral hemisphere can be involved in utero and that early impairment of the microvasculature can affect the normal development of the brain.
Case Report
This first-born male neonate was born at 39 weeks’ gestational age after an uventful pregnancy, with a weight of 3100 g, head circumference of 33 cm, and Apgar score of 10 at 1 and 5 minutes. From birth, a typical port-wine stain involving the left frontal scalp and the internal middle part of the upper eyelid was observed, together with asymmetry of the palpebral fissures and a small left eye. At 3 months of age, a slight asymmetry in the tonus in the upper limbs lead to cerebral imaging (Fig 1). At 9 months of age, infantile cerebral hemiplegia became prominent, with upper limb predominance. At 20 months of age, the child did not use his right hand except for rudimentary tasks, and it had gross stereognostic disturbances. Partial right hemianopsia appeared clinically probable. The patient’s nonmotor development was considered to be in the lower part of the normal range. He was able to use about 10 words, turn the pages of a magazine, and indicate images of interest with his right index finger. No seizures had been noted, including during febrile episodes. Clinical and MR findings confirmed that the left eye was slightly smaller, but ophtalmoscopic findings and visual acuity were normal. Electroencephalographic results obtained when the infant was aged 3 months showed an asymmetric amplitude and bursts of acute figures on the left side but no definite epileptic activity. At 20 months, the trace remained depressed and slow on the left side, but it was devoid of any epileptic activity. The karyotype was normal, 46XY.
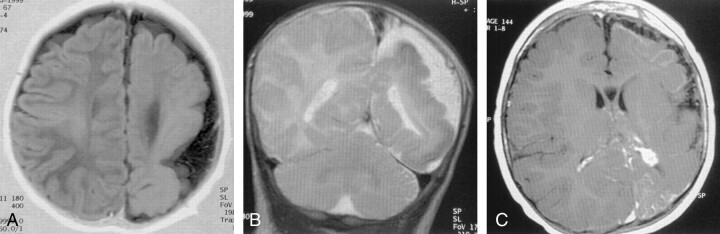
Fig 1.
MR images in a patient with SWD.
A, Axial inversion-recovery T1-weighted image obtained when the patient was aged 3 months shows a decrease in the size of the left hemisphere in the parietal region and a pachygyric appearance of the cortex.
B, Coronal T2-weighted image shows a pachygyric pattern in the left cortex, with irregularities in the cortical-white matter junctions.
C, Coronal T2-weighted image obtained after the injection of gadolinium-based contrast agent when the patient was aged 20 months shows an enlarged left choroid plexus, enhancement of the pial angioma, and large subarachnoid spaces on the left side without noticeable ventricular dilatation.
MR images were obtained with a 1.5-T machine when the infant was aged 3 and 20 months of age by using T1- and T2-weighted sequences and T1-weighted sequences with gadolinium-enhancement. A decrease in the size of the left cerebral hemisphere, which was more obvious in the occipital and parietal lobes, was noted. In contrasting with the large subarachnoïd spaces on the left side, the lateral ventricles showed no obvious asymmetry (Fig 1A). In the parieto-occipital areas, the cortex appeared dysplastic, with increased thickness; prominent, coarse gyri; and smooth cortical–white matter junctions on images obtained with T1-weighted sequences (Fig 1A). On T2-weighted images, these cortical-white matter junctions had an irregular and undulating pattern (Fig 1B). After the injection of gadolinium-based contrast material, diffuse pial enhancement appeared over the left hemisphere, and a marked enlargement of the ipsilateral choroid plexus was noted; this is typical of SWD angiomatosis (Fig 1C). At the second examination, the main change was in the appearance of low signal intensity on T2-weighted images of the superficial cortex in many of the pachygyric areas; this finding suggested the development of calcification.
Discussion
The intracranial vascular dysplasia in SWD consists of small tortuous venular structures that are predominant in the subpial compartment. The microvenular dysplasia results in abnormal venous drainage, which is mainly directed toward the deep venous structures of the brain (3). On early MR images, the brain parenchyma in usually has normal myelination or slight hypermyelination in the centrum semiovale on the side involved (1, 4). According to single photon emission CT (SPECT) results, cerebral blood flow is increased during the early asymptomatic period, whereas hypoperfusion and cerebral atrophy develop after the first hemiconvulsive episode or after the first year of life in children who do not have epileptic degradation (5). Thus, venous stasis and recurrent episodes of venular thrombosis are presumably the main factors responsible both for neurologic deterioration and postnatal hemispheric atrophy (6).
Both the clinical presentation of the present patient and the MR imaging findings suggest that the hemispheric lesions developed during the intrauterine period. As the timing of cell migration from the rhombencephalon toward the facial structures indicates, the developmental event responsible for SWD vascular dysplasia probably occurs 5–8 weeks after conception. In many instances, pachygyria, a macroscopic feature frequently associated with microscopic polymicrogyria or polymicrogyria visible on MR images (as in the present case), has been shown to be the result of microvascular disturbances that take place the main steps of cortical plate formation have occurred (7, 8). Thus, a plausible explanation for the present finding is that the abnormal venous drainage from the growing cortex may result in cortical rearrangements that eventually arrest hemispheric development.
Other genetic mechanisms are more speculative. SWD could be the consequence of a postmitotic genetic event involving clones of vascular cells devoted to particular cranial metameric segments. The SWD gene could also have a developmental function in other cell types. Thus, mutations extending to clones of nerve cells could result in a disorder of neuronal migration or cortical plate organization.
Although hemispheric disease in SWD is frequently complicated by severe, sometimes intractable epilepsy that starts in infancy (1), the child in the present case did not have seizures or paroxystic anomaly, as assessed with electroencephalography, during 2-year follow-up, despite the presence of severe cortical anomalies. Presumably, the degree of cortical maturation necessary for neuronal firing and epileptic initiation might be less easily achieved in certain early developmental lesions than in postnatal degenerative changes.
Cerebral imaging has become the usual practice in children with a port-wine stain in the metamer classically assigned to the V1 territory of cranial nerve V, but the timing and techniques that can provide the most useful information about the neurologic prognosis remain a matter of debate (9). To our knowledge, only one case with an unusual clinical presentation with early hemispheric atrophy has been reported (10) until now, but this case did not have evidence of cortical malformation. A better understanding of the frequency of cortical dysplasia associated with SWD vascular malformation is needed. For this specific purpose, early imaging may be useful in a neonate with a characteristic port-wine stain.
Acknowledgments
We are indebted to Luo Chao Bao, MD, for his help in digitalizing the pictures.
References
- 1.Arzimanoglou A, Aicardi J. The epilepsy of Sturge Weber syndrome: clinical features and treatment in 23 patients. Acta Neurol Scand 1992;140:18–22 [DOI] [PubMed] [Google Scholar]
- 2.Benedikt R, Brown D, Walker R. Sturge Weber syndrome: cranial MRI with Gd-DTPA. AJNR Am J Neuroradiol 1993;14:409–415 [PMC free article] [PubMed] [Google Scholar]
- 3.Lasjaunias P, ed. Vascular Diseases in Neonates, Infants and Children. Berlin, Germany: Springer-Verlag;1997. :435–439
- 4.Adamsbaum C, Pinton F, Rolland Y, et al. Accelerated myelination in early Sturge Weber syndrome: MRI-SPECT correlations. Pediat Radiol 1996;26:759–762 [DOI] [PubMed] [Google Scholar]
- 5.Pinton F, Chiron C, Enjolras, et al. Early SPECT in Sturge-Weber disease. J Neurol Neurosurg Psy 1997;63:616–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlos-Garcia J, Roach S, McLean WT. Recurrent thrombotic deterioration in the Sturge Weber syndrome. Child Brain 1981;8:427–433 [DOI] [PubMed] [Google Scholar]
- 7.Levine DN, Fisher MA, Caviness V. Porencephaly with microgyria: a pathologic study. Acta Neuropath 1974;29:99–113 [DOI] [PubMed] [Google Scholar]
- 8.Barkovitch AJ, Gressens P, Evrard P. Formation, maturation and disorders of brain neocortex. AJNR Am J Neuroradiol 1992;13:423–446 [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths D. Sturge Weber syndrome revisited: the role of neuroradiology. Neuropediatrics 1996;27:284–294 [DOI] [PubMed] [Google Scholar]
- 10.Campistol J, Garcia-Cazorla A, Gonzalez-Campo C. Sturge Weber disease with unusual cerebral atrophy and hydrocephalus. Eur J Paediat Neurol 1999;3:227–229 [Google Scholar]