Abstract
Simple Summary
In Adolescents and Young Adults (AYAs), fertility is an important factor for good quality of life. In the case of cancer of the female reproductive tract, treatment can impair fertility and therefore, AYAs may face the life-changing decision whether or not to undergo conservative, fertility-sparing cancer treatment. Solid evidence on the safety as well as reproductive outcomes of these treatments is necessary to provide patients the information they need to make a well-informed choice. This systematic review aims to provide an overview of the best evidence available on both oncological and reproductive outcome after various fertility-sparing surgical options in cervical, ovarian, and endometrial cancer.
Abstract
Fertility-sparing surgery (FSS) is increasingly being offered to women with a gynecological malignancy who wish to preserve fertility. In this systematic review, we evaluate the best evidence currently available on oncological and reproductive outcome after FSS for early stage cervical cancer, epithelial ovarian cancer, and endometrial cancer. An extensive literature search was conducted using the electronic databases Medline (OVID), Embase, and Cochrane Library to identify eligible studies published up to December 2020. In total, 153 studies were included with 7544, 3944, and 1229 patients who underwent FSS for cervical, ovarian, and endometrial cancer, respectively. We assessed the different FSS techniques that are available to preserve fertility, i.e., omitting removal of the uterine body and preserving at least one ovary. Overall, recurrence rates after FSS are reassuring and therefore, these conservative procedures seem oncologically safe in the current selection of patients with low-stage and low-grade disease. However, generalized conclusions should be made with caution due to the methodology of available studies, i.e., mostly retrospective cohort studies with a heterogeneous patient population, inducing selection bias. Moreover, about half of patients do not pursue pregnancy despite FSS and the reasons for these decisions have not yet been well studied. International collaboration will facilitate the collection of solid evidence on FSS and the related decision-making process to optimize patient selection and counseling.
Keywords: fertility-sparing surgery, conization, trachelectomy, hormonal therapy, cervical cancer, ovarian cancer, endometrial cancer, adolescents and young adults
1. Introduction
Each year, over 1,300,000 women are diagnosed with a gynecologic malignancy worldwide. Nearly 15% of these women are between 15 and 39 years of age [1]. This age group is often referred to with the term AYAs (Adolescents and Young Adults), although cut-offs for both minimum and maximum age of AYAs vary between studies and countries. Nowadays, 80% of AYA cancer patients survive their disease because of the improved early detection and advancements in cancer treatment of many cancer types [2]. As a result, the focus of oncologic treatment has expanded from survival only towards quality of life after surviving cancer [3]. Preservation of fertility is an important factor to achieve good quality of life in AYAs [4]. However, fertility can be impaired by surgery or the gonadotoxic effects of chemotherapy and radiotherapy. Fertility is highly at risk in the case of treatment of malignancies of the female genital tract, especially cervical, ovarian, and endometrial cancer. Standard treatment for these cancer types often includes hysterectomy and bilateral salpingo-oophorectomy and, depending on stage, (adjuvant) therapy in the form of pelvic radiation or chemotherapy. Fertility-sparing surgery (FSS), in which ovaries, uterus, and sometimes cervix are (partially) preserved, is being offered in selected cases, e.g., in women with early stage malignant cervical, ovarian, and endometrial tumors.
1.1. Cervical Cancer
Of all cervical cancers, 20% is diagnosed in AYAs, making cervical cancer the most common gynecologic malignancy and the second cause of cancer-related death in these young women [1]. Fortunately, incidence and mortality are declining in some areas of the world due to population screening and Human Papilloma Virus (HPV) vaccination. However, more than 110,000 AYAs are diagnosed with cervical cancer and over 31,000 AYAs still die of the disease each year worldwide [1]. (Radical) hysterectomy with or without pelvic lymphadenectomy is considered standard treatment for early stage cervical cancer (FIGO 2018 IA1-IB2) in women who do not want to have children anymore. In women with a strong desire to preserve fertility, FSS options include conization or simple trachelectomy (cone or barrel-shaped excision of the cervix without surgery of the parametrium), or (vaginal or abdominal) radical trachelectomy (removal of the cervix, parametrium, and upper vaginal cuff), leaving the uterine body intact. These fertility-sparing procedures are increasingly being offered, as more and more studies suggest acceptable oncological outcomes comparable to radical hysterectomy [5]. However, the pregnancy rates especially after abdominal radical trachelectomy (ART) are disappointing [6,7] The number of live births after ART in stage IB2 is only 9% [8]. The pregnancy rate after neoadjuvant chemotherapy (NACT) followed by conservative surgery is promising, but oncological safety is still unclear [6,9,10].
1.2. Ovarian Cancer
Ovarian cancer is the 4th most commonly diagnosed cancer in AYAs [1]. Epithelial ovarian cancer is the most common type, although non-epithelial ovarian cancer occurs more often in young women than in women over 40 years of age [2]. The incidence of ovarian cancer in AYAs represents 13% of all new diagnoses annually. The implication is that approximately 38,500 young women are diagnosed with ovarian cancer and that 10,000 of these women die from the consequences of the disease each year worldwide [1].
The standard management of clinical early stage epithelial ovarian cancer is surgical staging, which includes hysterectomy, bilateral salpingo-oophorectomy, omentectomy, peritoneal washings, and biopsies with or without pelvic and para-aortic lymph node sampling. Depending on final stage and histology, platinum-based adjuvant chemotherapy can be proposed. During FSS, removal of the contralateral ovary and uterus are omitted [11]. This conservative treatment is considered in AYAs with a strong desire to preserve fertility and with limited disease and no visible abnormalities during surgery. However, only observational, retrospective series comparing FSS and non-FSS in selected patients are available and controversy about women with high-risk prognostic factors remains [12,13].
1.3. Endometrial Cancer
Although only 4% of endometrial cancers occur in AYAs, this is still a global incidence of approximately 15,000 newly diagnosed women each year, of which 1600 young women die of the disease [1].
In endometrioid endometrial cancer, standard management involves total hysterectomy and bilateral salpingo-oophorectomy, leading to very high cure rates of 93% in low-risk disease [2]. The fertility-sparing alternative treatment includes hysteroscopic resection and/or curettage in combination with hormonal therapy with progestin. Complete remission rates of this fertility-sparing approach of 50–75% have been reported, demonstrating the clear concession to the high effectiveness of the standard treatment by hysterectomy [14,15,16]. Strict follow-up with hysteroscopic evaluation and endometrial sampling is advised.
The level of evidence on oncological safety and chance of successful pregnancy after FSS in patients with a gynecological malignancy is low, because it is mainly based on retrospective case series including small numbers of patients and events. Moreover, follow-up is often short and incidence of pregnancy and pregnancy outcome are incompletely reported. This complicates adequate counseling of AYAs with gynecological cancer and the wish to preserve fertility and consequently, hampers these women to make the life-changing choice they are facing.
In this review, we evaluate the best evidence currently available on oncological and reproductive outcome after FSS for early stage cervical cancer, epithelial ovarian cancer, and endometrial cancer.
2. Materials and Methods
2.1. Search Strategy
We performed a systematic review in accordance with the PRISMA guidelines. This review was registered in the International Prospective Register of Systematic Reviews (PROSPERO, number CRD42020173523). A literature search was conducted using the electronic bibliographic databases Medline (OVID), Embase, and the Cochrane Library to identify eligible studies published between January 2000 and March 2020 in English or Dutch language. We searched for keywords and equivalent words in the title/abstract and translated the search terms according to the standards of each database. Keywords for uterine cervical neoplasms, ovarian neoplasms, and endometrial neoplasm were combined with terms for fertility-sparing treatments in general and trachelectomy specifically. The search string is presented in Table S1. Reference lists of the included studies and retrieved review articles were searched to identify relevant articles not found in the initial search. Searches were re-run before the final analysis on the 3rd of December 2020.
2.2. Study Selection
The articles retrieved during the searches were screened for relevance on title/abstract and subsequently full text by two authors independently. Discrepancies were resolved by consensus after assessment by a third author. Rayyan, a systematic review web application, was used as a tool to screen the articles and to ensure the authors were blinded to each other’s decisions.
Included articles needed to specify oncological and/or reproductive outcomes after FSS, i.e., conization or (vaginal or abdominal) trachelectomy in cervical cancer, surgical staging with preservation of uterus and at least one ovary in epithelial ovarian cancer, and uterine preservation combined with hormonal therapy in endometrial cancer. Data published regarding fertility-sparing cancer treatment during pregnancy, in childhood cancer, or in non-epithelial ovarian cancer were excluded from our analysis.
The following studies were also excluded: (1) review articles without any new patient data, (2) case reports or small case series with less than 20 patients, (3) letters to editors, commentaries, or (conference) abstracts. Of the articles with duplicate patient information and articles updating prior published series, the articles with the most recent and complete data were included.
2.3. Data Extraction
A pre-defined data extraction form was used by three authors independently to collect and record the data of the selected studies. For each study, data regarding study characteristics (first author, year of publication, country), study design (type of study, data source, inclusion period, sample size), participants and tumor characteristics (age, tumor histology, FIGO stage), and intervention and outcome details were extracted.
2.4. Quality Assessment
The quality of the included articles was assessed using the Newcastle–Ottawa scale (NOS), which is suitable for assessing the quality of a non-randomized cohort and case–control studies [17]. This scale contains 8 items within 3 domains (selection, comparability, and exposure or outcomes) and the total maximum score is 9. A study receiving a score of 5 or more was considered as high quality and included in the final analysis. Three authors were involved in the quality assessment. The score of all initially included studies is presented in Tables S2–S4.
2.5. Data Synthesis and Analysis
Descriptive statistics were used to analyze the frequencies, mean and median values of demographic data, and oncological and reproductive outcomes. Pearson’s chi-square test or Fisher’s exact test was used to compare the oncological outcome between subgroups. p < 0.05 was considered statistically significant.
3. Results
3.1. Study Characteristics
Of the 5598 screened articles, 153 met the predefined inclusion criteria and were assessed for their quality (Figure 1) [8,14,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168]. A total of 16 studies (5 cervical, 3 ovarian, and 8 endometrial cancer) were excluded for final analysis due to a NOS score of 4 or less (Tables S2–S4). All but 2 included studies were cohort studies, of which 16 had a prospective and 119 a retrospective design (of which 8 analyzed prospectively collected data). There was one matched case–control study on cervical cancer and one randomized controlled trial on endometrial cancer. Patients were recruited from medical records (n = 129) or national registries (n = 8). The included studies and their characteristics and outcome measures are listed in Tables S5–S11.
Figure 1.
PRISMA flowchart summarizing the process for the identification of eligible articles.
3.2. Patient Characteristics and Treatment Modalities
The total study population of this review consists of 7544 women with cervical cancer, 3944 with epithelial ovarian cancer, and 1229 with endometrial cancer. The median ages are 31.6, 29.0, and 32.6 years, respectively. The histology, stage, and treatment modalities per tumor type are shown in Table 1, Table 2 and Table 3.
Table 1.
Main characteristics of patients with cervical cancer undergoing FSS.
| LLETZ/CKC/ST | VRT | ART | ||
|---|---|---|---|---|
| Laparotomic | MIS | |||
| Study/patient characteristics | ||||
| n of studies | 29 | 31 | 28 | 10 |
| Total n of patients | 2540 | 2477 | 2268 | 566 |
| n of patients excluded # | 108 | 76 | 91 | 32 |
| Age (y), median (range) | 32 (22–46) | 31 (21–42) | 32 (22–40) | 31 (22–40) |
| Tumor characteristics ^ | n of patients (%) | n of patients (%) | n of patients (%) | n of patients (%) |
| FIGO stage * | ||||
| IA1 | 1551 (60.4) | 248 (9.0) | 165 (7.1) | 13 (2.4) |
| IA2 | 547 (21.3) | 311 (11.3) | 218 (9.4) | 50 (9.3) |
| IA-IB | 0 | 230 (8.4) | 143 (6.2) | 0 |
| IB1 | 452 (17.6) | 1933 (70.3) | 1723 (74.6) | 453 (84.0) |
| IB2 | 18 (0.7) | 3 (0.1) | 20 (0.9) | 3 (0.6) |
| IB nos | 0 | 0 | 17 (0.7) | 19 (3.5) |
| IIA | 2 (0.1) | 26 (0.9) | 25 (1.1) | 1 (0.2) |
| Histological subtype | ||||
| SCC | 2316 (82.2) | 1906 (67.9) | 1689 (72.4) | 339 (63.2) |
| AC | 467 (16.6) | 770 (27.4) | 518 (22.2) | 169 (31.5) |
| ASCC | 25 (0.9) | 86 (3.1) | 84 (3.6) | 12 (2.2) |
| Other | 8 (0.3) | 47 (1.7) | 43 (1.8) | 16 (3.0) |
| LVSI~ | 150/784 (19.1) | 586/1877 (31.2) | 657/1628 (40.4) | 59/341 (17.3) |
FSS—fertility-sparing surgery; LLETZ—large loop excision of transformation zone; CKC—cold knife conization; ST—simple trachelectomy; VRT—vaginal radical trachelectomy; ART—abdominal radical trachelectomy; MIS—minimal invasive surgery (laparoscopic or robot-assisted); SCC—squamous cell carcinoma; AC—adenocarcinoma; ASCC—adenosquamous cell carcinoma; LVSI—lymph-vascular space invasion; nos—not otherwise specified; # No FSS due to positive lymph nodes/margins or other reasons contraindicating FSS; ^ In some studies, this also includes excluded patients or patients who underwent other types of FSS if data were not reported separately; therefore, the n of patients could exceed the total n of patients; * FIGO 2009 classification was used in all but one study, which used FIGO 2018 (n = 32); ~limited to publications with available data.
Table 2.
Main characteristics and treatment modalities of patients with epithelial ovarian cancer undergoing FSS.
| Patient Characteristics | |
|---|---|
| Total n of patients | 3944 |
| Age (y), median (range) | 29 (23.5–36.5) |
| Tumor characteristics | n of patients (%) |
| FIGO stage | |
| IA | 912 (23.1) |
| IB | 9 (0.2) |
| IA/IB | 1154 (29.3) |
| IA/IC1 | 18 (0.5) |
| IC nos | 808 (20.5) |
| IC1 | 220 (5.6) |
| IC2 | 35 (0.9) |
| IC3 | 25 (0.6) |
| I nos | 9 (0.2) |
| ≥II | 646 (16.4) |
| na | 108 (2.7) |
| Histological subtype | |
| mucinous | 1775 (45.0) |
| endometrioid | 1059 (26.9) |
| serous | 760 (19.3) |
| clear cell | 287 (7.3) |
| mixed | 15 (0.4) |
| other/na | 48 (1.2) |
| Grade | |
| 1–2 | 3015 (76.4) |
| 2–3 | 6 (0.2) |
| 3 (+clear cell) | 855 (21.7) |
| 3 non-clear cell | 568 (14.4) |
| na | 68 (1.7) |
| Treatment modalities * | n/total (%) |
| Type of FSS | |
| staging | |
| Complete # | 776/1110 (69.9) |
| LND ^ | 617/1110 (55.6) |
| Surgical technique | |
| laparotomy | 275/426 (64.6) |
| laparoscopy | 151/426 (35.4) |
| Chemotherapy | |
| adjuvant | 1815/3586 (50.6) |
FSS—fertility-sparing surgery; LND—lymph node dissection; nos—not otherwise specified; na—not available; * limited to publications with available data; # unilateral salpingo-oophorectomy or cystectomy, cytology, biopsies, and omentectomy; ^ pelvic and/or para-aortic.
Table 3.
Main characteristics and treatment modalities of patients with endometrial cancer undergoing FSS.
| Patient Characteristics | |
|---|---|
| Total n of patients | 1229 |
| Age (y), median (range) | 32.6 (31–36.1) |
| Tumor characteristics | n of patients (%) |
| FIGO stage | |
| IA | 1020 (83.0) |
| IB | 6 (0.5) |
| IB-IIA | 15 (1.2) |
| I nos | 188 (15.3) |
| Histological subtype | |
| endometrioid | 1110 (90.3) |
| adenosquamous | 10 (0.8) |
| mucinous | 1 (0.1) |
| mixed | 8 (0.7) |
| adenocarcinoma nos | 35 (2.8) |
| na | 65 (5.3) |
| Grade | |
| 1 | 1136 (92.4) |
| 2 | 86 (7.0) |
| 3 | 4 (0.3) |
| na | 3 (0.2) |
| Treatment modalities * | n of patients (%) |
| Type of FSS | |
| D&C | 245 (19.9) |
| HR | 155 (12.6) |
| HR + D&C | 57 (4.6) |
| NR | 772 (62.8) |
| Hormonal therapy | |
| MPA | 458 (37.3) |
| MA | 337 (27.4) |
| LNG-IUD | 91 (7.4) |
| MPA + MA | 7 (0.6) |
| MPA/MA + LNG-IUD | 26 (2.1) |
| MPA/MA + metformin | 57 (4.6) |
| MPA/MA nos | 198 (16.1) |
| Progestin nos | 51 (4.1) |
| Other ^ | 4 (0.3) |
FSS—fertility-sparing surgery; HR—hysteroscopic resection; D&C—dilatation and curettage; MA—megestrol acetate; MPA—medroxy-progesterone acetate; LNG-IUD—levonorgestrel intrauterine device; nos—not otherwise specified; na—not available; NR—not reported; * Limited to publications with available data; ^ norethisterone acetate (n = 2) and hydroxyprogesterone caproate (n = 2).
In patients with cervical cancer, squamous cell carcinoma (SCC) was the most common subtype. The majority of patients were diagnosed with stage IB1 (FIGO 2009) disease. FSS consisted of simple or radical trachelectomy, conization or large loop excision of the transformation zone (LLETZ), with or without pelvic lymphadenectomy. In 12 studies, patients received NACT prior to FSS. The majority of patients underwent an LLETZ, conization, or simple trachelectomy (ST). In total, 3.9% of patients (307/7851) initially planned for FSS had positive lymph nodes, positive resection margins, or other reasons contraindicating FSS. These patients underwent non-FSS treatment. In 2018, the FIGO staging for cervical cancer was revised [169]. All but one study ([26], n = 32) in this review used the former FIGO 2009 classification.
The majority of patients with epithelial ovarian cancer had a low-grade tumor (76.7%), mucinous histology (45%), and was diagnosed with stage IA or IB (52.6%). FSS consisted of unilateral salpingo-oophorectomy and preservation of the uterus and contralateral ovary, with or without additional staging procedures. In 18 studies, the completeness of surgical staging was specified. Complete peritoneal staging including peritoneal washings and/or biopsies and omentectomy ranged from 41 to 100% with an average of 70% (776/1110 patients). Pelvic and/or para-aortic lymph node sampling was performed in 56% (617/1110 patients). Of the patients with known details about the surgical technique, 275 of 426 (64.6%) underwent a laparotomy and 151 of 426 (35.4%) a laparoscopy. In total, 1815 of 3586 patients (50.6%) received adjuvant chemotherapy.
Almost all patients with endometrial cancer were diagnosed with grade 1 (92.4%), endometrioid adenocarcinoma (90.3%), and stage IA (83%). In 14 studies, the type of FSS prior to the start of hormonal therapy was reported and consisted of hysteroscopic resection (HR) and/or dilatation and curettage (D&C). In all 23 included studies, patients received hormonal therapy with progestin, although type, dosage, and treatment scheme differed considerably between studies. The majority of patients were treated with medroxyprogesterone acetate (MPA) or megestrol acetate (MA). MPA was used in 14 studies, all with different dosages, ranging from 20 to 1500 mg/day. In 17 studies, MA was used, in most patients with a dosage of 160 mg/day (range 40–800 mg/day). Patients received a levonorgestrel intrauterine device (LNG-IUD, 20 or 52 mg) as a single treatment in 5 studies (n = 91) and it was used in combination with oral progestin in 6 studies (n = 45). In 2 studies, metformin was given in addition to oral progestin and in another 2 studies, progestin was combined with GnRH analogues. The median duration of hormonal therapy with oral progestin ranged from 4.2 to 10.9 months and with LNG-IUD from 9.1 to 25.8 months.
3.3. Oncological Outcome
In Table 4, the oncological outcome after FSS in each of the gynecological malignancies is presented. Not all studies reported both recurrence and death from disease.
Table 4.
Oncological and reproductive outcome after FSS in patients with cervical, ovarian, and endometrial cancer.
| Cervical Cancer | Ovarian Cancer | Endometrial Cancer | ||||
|---|---|---|---|---|---|---|
| LLETZ/CKC/ST | VRT | ART | ||||
| Laparotomic | MIS | |||||
| Oncological outcome * | ||||||
| Recurrence | 28/785 (3.6) | 82/1973 (4.2) | 47/1520 (3.1) | 15/335 (4.5) | 171/1084 (15.7) | 297/855 (34.7) |
| Died of disease | 6/785 (0.8) | 34/1973 (1.7) | 23/1520 (1.5) | 7/479 (1.5) | 489/3318 (14.7) | 5/648 (0.8) |
| Complete response | – | – | – | – | – | 736/918 (80.2) |
| Persistent disease | – | – | – | – | – | 182/918 (19.8) |
| PR | – | – | – | – | – | 16/504 (3.2) |
| SD | – | – | – | – | – | 52/504 (10.3) |
| PrD | – | – | – | – | – | 28/504 (5.6) |
| Follow-up in months, | 53 (9–137) | 52 (9–131) | 47 (12–120) | 44 (10–98) | 66 (38–143) | 56 (17–92) |
| median (range) | ||||||
| Fertility outcome * | ||||||
| Pregnancy wish ^ | 148/305 (48.5) | 599/1089 (55.0) | 510/1062 (48.0) | 71/199 (35.7) | 148/335 (44.2) | 279/446 (62.6) |
| Pregnancies ^ | 241/612 (39.4) | 707/1539 (45.9) | 353/1635 (21.6) | 81/344 (23.5) | 280/750 (37.3) | 256/505 (50.7) |
| Pregnant patients ^ | 196/665 (29.5) | 512/1392 (36.8) | 255/1427 (17.9) | 58/317 (18.3) | 207/701 (29.5) | 261/708 (36.9) |
| Pregnancy rate # | 82/138 (59.4) | 361/546 (66.1) | 212/471 (45.0) | 42/71 (59.2) | 110/148 (74.3) | 161/241 (66.8) |
| Fetal loss ~ | 36/234 (15.4) | 147/686 (21.4) | 101/407 (24.8) | 19/81 (23.5) | 17/191 (8.9) | 67/214 (31.3) |
| Termination ~ | 7/234 (3.0) | 26/686 (3.8) | 20/407 (4.9) | 0/81 (0) | 3/191 (1.6) | 0/214 (0) |
| Live birth ~ | 181/234 (77.4) | 484/686 (70.6) | 236/407 (58.0) | 58/81 (71.6) | 236/264 (89.4) | 172/239 (72.0) |
| Term delivery $ | 111/181 (61.3) | 262/484 (54.1) | 80/236 (33.9) | 26/58 (44.8) | 161/164 (98.2) | 82/93 (88.2) |
| Preterm delivery $ | 27/181 (14.9) | 146/484 (30.2) | 109/236 (46.2) | 32/58 (55.2) | 3/164 (1.8) | 9/93 (9.7) |
| Not specified | 43 | 76 | 47 | 0 | 72 | 79 |
| Pregnancy ongoing | 10 | 20 | 25 | 4 | 7 | 2 |
n/total (%); ^ total patients; # total patients with pregnancy wish who succeeded; ~ total pregnancies; $ total live births; LLETZ—large loop excision of the transformation zone; CKC—cold knife conization; ST—simple trachelectomy; VRT—vaginal radical trachelectomy; ART—abdominal radical trachelectomy; MIS—minimal invasive surgery (laparoscopic or robot-assisted); PR—partial response; SD—stable disease; PrD—progressive disease; * Limited to publications with available data.
In 29 studies on cervical cancer, involving 2432 patients, LLETZ, conization, or ST with or without pelvic lymphadenectomy was performed. In total, 28 (3.6%) patients had recurrent disease and 6 (0.8%) died of disease during a median follow-up of 53 months (range 9–131 months). More detailed information was available of 23 recurrences. Significantly more recurrences occurred in patients with FIGO stage IB1 (3.1%) and IB2 (5.6%) disease than in patients with FIGO stage IA1 (0.2%) and IA2 (0.7%) disease. SCC recurred in 0.7% of patients, adenocarcinomas (AC) in 1.1%, and adenosquamous cell carcinomas (ASCC) in 4%. The differences between these histological subtypes are statistically not significant (p = 0.159).
In 31 studies, including 2401 patients, a vaginal radical trachelectomy (VRT) was performed. Recurrent disease occurred in 82 (4.2%) patients and 34 (1.7%) patients died, after a median follow-up of 52 months (range 9–131 months). Unfortunately, several studies did not report on tumor size and LVSI, or combined stage IA and IB1 disease, which are factors associated with recurrent disease. In 7 studies (n = 468 patients) reporting on recurrence rates, details on initial tumor size were described. The overall recurrence rate was 6.2%, divided into 3.5% in patients with tumors <2 cm and 20.5% in ≥2 cm (p ≤ 0.001). None of the patients with tumors <2 cm received NACT, although 5 of 83 patients with a tumor size ≥2 cm received NACT. In 12 studies (n = 1134), detailed information on recurrence according to histological subtype was described. The overall recurrence rate was 4.1%. In patients with SCC or AC, it was 3.4% and 6.1%, respectively (p = 0.03). In only 5 studies (n = 656), detailed information on initial LVSI in patients who had recurrent disease was reported. LVSI was found in 31.3% of patients. The overall recurrence rate was 3.8%. In tumors with LVSI vs. tumors without LVSI, recurrence rate was 5.1% vs. 3.0%, respectively (p = 0.16).
The abdominal approach for radical trachelectomy is more radical in terms of parametrial and paracervical resection compared to the vaginal approach. This procedure was originally offered to patients with less favorable prognosis, e.g., tumors >2 cm and LVSI. ART can be performed by laparotomy or as minimal invasive (laparoscopic or robot-assisted) procedure. In 28 studies, 2177 patients underwent ART per laparotomy. Recurrent disease was diagnosed in 47 (3.1%) patients and 23 (1.5%) deaths were reported. Median follow-up was 47 months (range 12–120 months). In 8 studies, more detailed information on recurrences was described. Based on these studies (n = 863 patients), recurrences were seen in 2.0% of patients with SCC, in 4.1% with AC, and in 2.7% with ASCC. These differences were not significantly different. Only one study found significantly more recurrences in ASCC [56]. In 3 studies (n = 367), detailed information was described on recurrences (n = 11) and tumor size. In total, 11 patients with tumors ≥2 cm received NACT. Overall, in 3.0% of patients, a recurrence occurred of 2.3% in patients with tumors <2 cm and 3.6% with tumors ≥2 cm (p = 0.74).
In 10 studies, involving 534 patients, a minimal invasive approach for ART was used. Recurrence and death rates were reported in 9 studies, with a median follow-up of 44 months (range 10–98 months). In total, 15 (4.5%) recurrences occurred and 7 (1.5%) patients died of disease. In 4 studies, including 177 patients, more detailed information on recurrences (n = 14) was reported, which were significantly more often found in patients with ASCC (28.6%) than with AC (4.2%) or SCC (7.3%). Almost all recurrences (n = 12) occurred in patients with FIGO stage IB1 disease. In 2 studies with 106 patients, tumor size was specified. In 4.3% of tumors <2 cm, recurrences occurred, compared to 18.9% of tumors ≥2 cm (p = 0.03).
Some studies (n = 24, including 3363 vs. 16,405 patients) compared oncological outcome after FSS with non-FSS (Table S8). The disease-free survival (DFS) and overall survival (OS) or disease-specific survival (DSS) were not significantly different in both groups. In 15 studies, with 830 patients undergoing FSS and 1030 patients undergoing non-FSS, recurrences occurred in 2.4% vs. 3.3% patients, respectively. In one study, a trend was seen towards a worse DSS in FSS (conization or radical trachelectomy, n = 125) versus non-FSS (n = 2592) in patients with FIGO stage IB1 tumors >2 cm (82.4% vs. 90.4%, p = 0.112) [99].
In 21 of 24 studies with epithelial ovarian cancer patients, recurrence rates were reported and another 21 studies described death rates, with a median follow-up of 66 and 67 months (range 38–143), respectively.
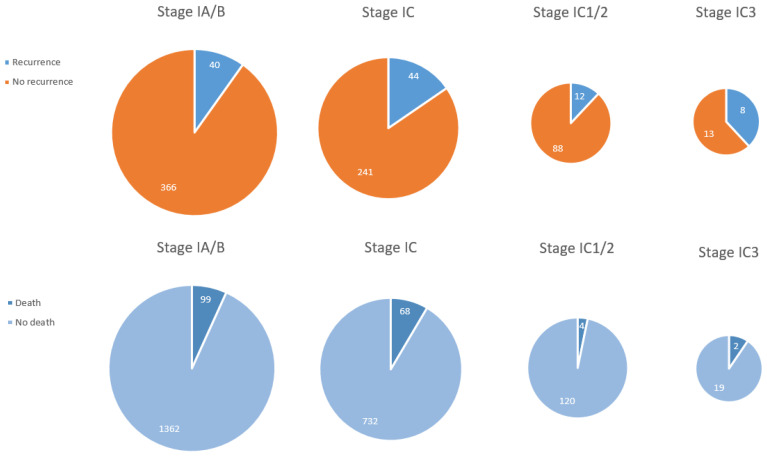
In 15 studies, data about disease stage in relapsing patients were provided (Figure 2). In total, 40 of 406 patients (9.9%) with stage IA/IB and 44 of 285 patients (15.4%) with stage IC had a recurrence (p = 0.027). Stage IC is divided into three prognostic categories (IC1, IC2, and IC3) since the revised FIGO staging of ovarian cancer in 2014 [170]. Only 8 studies used this latest FIGO staging showing that 12 of 100 patients (12%) with stage IC1/2 and 8 of 21 patients (38.1%) with stage IC3 relapsed. This difference in recurrence rate is statistically significant (p = 0.003). The reported number of patients undergoing FSS with stage II or higher is extremely low, but 4 of the reported 10 patients (40%) with stage ≥II (stage II n = 2, stage III n = 2) relapsed compared to 88 of 639 patients (13.8%) with stage I (p = 0.040).
Figure 2.
Recurrence rate and death rate in epithelial ovarian cancer per FIGO stage.
Death rate was 6.8% (99/1461) in stage IA/B versus 8.5% (68/800) in stage IC (p = 0.134), 3.2% (4/120) in stage IC1/2 versus 9.5% (2/21) in stage IC3 (p = 0.209), and 7.2% (168/2154) in stage I versus 39.2% (250/387) in stage ≥II (p ≤ 0.001) (Figure 2).
In 11 studies, the grade of the carcinomas in patients who suffered from recurrences was described and complete data were reported. In 62 of 622 (9.1%) patients with grade 1 or 2 carcinoma and in 32 of 125 (25.6%) patients with grade 3 carcinoma (including clear cell histology), the cancer recurred (p ≤ 0.001). From data of 10 studies, grade 3 non-clear cell histology could be analyzed separately. These studies showed a recurrence rate of 55% (11 of 20 patients, p ≤ 0.001).
Death rate was 8.8% (219/2489) in grade 1–2 carcinomas. This is statistically significantly lower compared to grade 3 (including clear cell) carcinomas (213/711, 30.0%, p ≤ 0.001), but not to grade 3 non-clear cell carcinomas (5/26, 19.2%, p = 0.063). A high percentage (40%) of stage II or higher with worse prognosis was included in the group of grade 3 carcinomas, including clear cell histology, influencing this subgroup analysis. When limiting these calculations to stage I disease, death rate was 5.8% (115/1987) in grade 1–2 carcinomas, which is statistically significant for grade 3 (including clear cell) carcinomas (14.1%, 57/405, p ≤ 0.001) but not for grade 3 non-clear cell carcinomas (12.5%, 2/16, p = 0.239).
In total, 12 of 85 patients (14.1%) with clear cell carcinoma relapsed compared to 82 of 673 (12.2%) patients with non-clear cell carcinoma (p = 0.610). Death rates were also not statistically different (5/106, 4.7% vs. 55/933, 5.9%, respectively, p = 0.622).
Often the localization of the recurrence was not reported. In 67 patients with known location of the recurrence, an isolated ovarian tumor was found in 23 patients (34.3%) and other sites of recurrence with or without involvement of the remaining ovary in 44 patients (65.7%). Recurrences occurring in patients with initially low-risk tumors (stage IA, grade 1–2, or non-clear cell histology) were more frequently isolated (62%). There were no recurrences recorded involving the uterus.
Some studies (n = 13) compared oncological outcome after FSS with radical, non-FSS (Table S10). The recurrence rate of all patients who underwent FSS was 15.7% (171/1084) compared to 18.5% (266/1436) after non-FSS (p ≤ 0.001). Death rates were also lower in FSS, with 14.7% (489/3318) after FSS and 21% (1841/8774) after radical surgery (p ≤ 0.001).
In 21 of 23 studies on endometrial cancer, the response rate was reported with a median follow-up of 53.8 months. Complete response (CR) occurred in 80.2% of patients (736/918). The median time to CR was 5.5 months (range 3–9 months, reported in 17/21 studies). The remaining patients (182/918, 19.8%) had persistent disease. In 13 of the 21 studies (n = 504), the response in these patients was further divided into partial response (n = 16, 3.2%), stable disease (n = 52, 10.3%), and progressive disease (n = 28, 5.6%).
In 21 studies, recurrence rates in patients with initial CR were described, which was 34.7% (297/855). The 5-year recurrence-free survival (RFS) ranged from 33 to 68% in 5 studies. Although death rate was reported in 12 studies, in only 1 study, deaths occurred (5/648, 0.8%).
The study by Chung et al. is the only study in which a new classification of endometrial cancer, based on molecular subtypes, was used [143]. This ProMisE classification distinguishes endometrial cancer with (1) mismatch repair deficiency (MMRd), (2) DNA polymerase epsilon (POLE) mutation, (3) wild-type p53 (p53wt), and (4) abnormal p53 (p53abn) [171]. Of the 57 patients included in this study, 9 (15.8%) had a tumor with MMRd, 2 (3.5%) had a tumor with POLE mutation, 45 (78.9%) tumors were p53wt, and 1 tumor (1.8%) had p53abn. The overall CR rate to hormonal therapy was 75.4%. Patients with MMRd tumors had a significantly lower complete or partial response rate than those with p53wt (44.4% vs. 82.2%, p = 0.018). There was no difference in recurrence rate after achieving CR between patients with MMRd and p53wt (25.0% vs. 43.2%, p = 0.629). The oncologic outcomes were not compared for POLE and p53abn subtypes because of the small number of patients.
Only one study, by Greenwald et al., compared hormonal therapy with primary surgery, using the Surveillance, Epidemiology, and End Results (SEER) database [149]. Cancer-specific death rates were 3.1% (5/161) and 0.7% (46/6178), respectively (p = 0.001). However, when comparing to a propensity score-matched cohort (1/161, 0.6%), the difference was not statistically significant (p = 0.099). Recurrence rates were not reported.
In 2 studies, patients with grade 2 or 3 carcinoma were included and data about grade in patients who had CR were provided. Of 45 patients with grade 2–3 carcinoma, 37 (82.2%) had CR compared to 531 of 659 patients (80.6%) with grade 1 carcinoma (p = 0.787). Of these 37 patients, 15 (40.5%) had a recurrence compared to 188 of 509 patients (36.9%) with grade 1 carcinoma and CR (p = 0.661).
In 3 studies, LNG-IUD as a single treatment was used. The CR rate with LNG-IUD was 84% (63/75) versus 78.9% (435/551) with oral progestin (p = 0.309). The recurrence rate in these patients with CR was 9.5% (6/63) with LNG-IUD compared to 45.7% (217/475) with oral progestin (p ≤ 0.001). There was no difference in treatment effect between 20 or 52 mg LNG-IUD, although numbers were small. Due to heterogeneity in treatment strategies and lack of available data, no adequate subgroup analysis could be performed on type or dosage of oral progestin therapy.
3.4. Reproductive Outcome
Table 4 shows the reproductive outcome after FSS per tumor type. Most studies did not report if patients had a wish to conceive and attempted pregnancy after they underwent FSS. Of the studies that did, 51% of patients with a gynecological malignancy had a pregnancy wish after FSS.
In patients with cervical cancer, 36–55% reported a pregnancy wish after FSS. A vaginal approach of FSS in cervical cancer is thought to have the best reproductive outcome, possibly due to less extensive resection of the cervix/parametrium. In patients who underwent LLETZ, conization, or ST, the pregnancy rate was 59%, with a live birth rate of 77%. After VRT, the pregnancy rate and live birth rate were 66% and 71%, respectively. The lowest pregnancy rate (45%) and live birth rate (58%) were found after ART by laparotomy, which is significantly lower than other types of FSS. In the minimal invasive approach, there was a pregnancy rate of 59%, with a live birth rate of 72%.
After the least radical FSS (LLETZ, conization, or ST), fetal loss was 15%, which is significantly lower than after VRT (21%), laparotomic ART (25%), and minimal invasive ART (24%). Furthermore, the risk of preterm delivery was also significantly lower and was 15%, 30%, 46%, and 55%, respectively.
In epithelial ovarian cancer, pregnancy wish after cancer treatment was reported in 6 studies and ranged from 25 to 65.4%, with an average of 44.2%. The live birth rate varied from 57.1 to 100%.
In 12 studies on endometrial cancer, 62.6% of patients with CR on hormonal therapy had a pregnancy wish (range 36.7–100%). Of the patients with CR, 36.9% became pregnant (reported in 17 studies).
4. Discussion
In this study, we summarized and analyzed the available literature on FSS in gynecological cancers known to date and performed a critical quality assessment, resulting in this overview of fertility-sparing options in three different types of gynecological malignancies.
Mostly, retrospective cohort studies were found describing a heterogeneous patient population. Next to that, information on recurrences and deaths are often missing or not reported per subgroup, which complicates comparison between studies and groups of patients. With its limitations in mind, some important conclusions can be drawn from our analysis.
One of the most important conclusions is that we need a structured, well-developed international database with predefined variables before we can optimally counsel our patients based on solid data.
A second conclusion is that many of the patients that undergo FSS to preserve fertility choose not to achieve a pregnancy after treatment. This decision is likely to be influenced by many factors, not all known before treatment. However, previous reports on fertility preservation in cancer patients undergoing ovarian tissue cryopreservation also showed that only a minority of women will use the offered possibility of achieving pregnancy this way [172]. Apparently, this is also true for gynecological cancer as only 44–63% of the patients in our analysis were shown to have a pregnancy wish after treatment. It is important to obtain more insight into the reasons women undergo FSS procedures, thereby deviating from standard treatment and sometimes accepting increased risk of recurrence or death, and not pursuing pregnancy even if their outcome is reassuring.
A third conclusion we can draw is that in general, the recurrence rate after FSS for gynecological cancer is small, sometimes even smaller than after standard treatment, but selection of patients with good prognosis is likely. Therefore, conclusions should be generalized with caution.
In this systematic review, we have found that in patients with early stage cervical cancer (majority FIGO 2009 stage IA-IB1), the chance for recurrence after FSS varies between 3.1 and 4.5% after the several evaluated treatment modalities and 0.8–1.7% died of their disease. Two groups showed worrying recurrence rates: women with carcinomas ≥2 cm treated with VRT (20.5%) or with minimal invasive ART (18.9%). Internationally, the chance for recurrence after non-FSS in early stage cervical cancer is reported to be 6–9% [173,174]. In some studies included in this review, which provided data on both FSS and non-FSS, recurrence and death rate in both groups are not reported to be different. The risk of recurrence in cervical carcinoma is mostly influenced by tumor size, nodal status, and tumor characteristics, such as deep stromal invasion [175]. In the studies included in our review, information on these prognostic factors is sporadically given. Since FSS may harbor an increased risk for recurrence due to less radical treatment, one can imagine that patients with poor prognostic factors are offered FSS less frequently. The reassuring outcomes we found regarding the risk of recurrence may reflect a good selection of patients with favorable prognostic factors rather than the oncological safety of FSS itself.
Our review shows that ART per laparotomy has the lowest pregnancy rate (45.0%) compared to all other treatment modalities with pregnancy rates around 60%. As expected, the radicality of cervical surgery is related to the risk of preterm delivery. As more radical surgery is performed in larger sized cervical tumors, treatment with NACT to reduce tumor size, enabling less radical surgery in larger tumors, may be beneficial in reducing the risk of prematurity. The safety and feasibility of this combined treatment for patients with FIGO 2018 IB2 cervical carcinoma is now studied in the prospective CONTESSA/NEOCON-F trial and results are awaited [10].
In ovarian cancer, the same phenomenon of favorable patient selection is seen. Prognostic factors include stage, histological subtype, and grade. Unfortunately, most studies are too small to allow reliable subgroup analysis. The majority of patients in our review had stage 1A or IB epithelial ovarian cancer with a recurrence rate of 9.9%. Intra-abdominal spill or ascites (stage IC) significantly increased the risk of recurrence to 15.4%. However, many studies did not use the new FIGO staging classification that further subdivides stage IC into stage IC1, IC2, and IC3. This discrimination is important as we found 38.1% recurrences in stage IC3 vs. 12% in stage IC1/2. As the number of available patients for analysis was minimal (n = 21), the controversy on whether it is safe to offer FSS to young women with stage IC continues. Given the tendency of ovarian cancer to recur transperitoneally, it is hypothesized that grade and stage, but not FSS, add to the risk of relapse.
The incidence of different histological subtypes in AYAs is different from older women. Non-epithelial histology occurs more often in young women. We did not include these malignancies in our review because of their different biological behavior and often more favorable prognosis. This is why there is a general acceptance to offer FSS in these women in cases of low-stage disease. Inclusion in the current analysis would have clouded our results. The majority of patients in our review had mucinous histology and numbers of patients with serous or clear cell histology were much lower. Nowadays, mucinous tumors are divided into expansile vs. infiltrative types. Only one small study discriminated between these types and found no difference in recurrence rate. Adherence to the new WHO classification system for ovarian cancer is necessary to learn whether FSS for all mucinous tumors is safe.
In epithelial ovarian cancer, grade is very important in estimating the chance of recurrence. FSS for high-grade tumors cannot be safely advised as we found 25.6% recurrences in grade 3 carcinomas (including clear cell histology) compared to 9.1% in grade 1–2. It is unlikely that the high recurrence rate in grade 3 carcinomas is only explained by inclusion of clear cell histology, as in our analysis, the recurrence rate in clear cell carcinoma was 14.1% versus 12.2% in non-clear cell carcinoma. Unfortunately, most studies do not report detailed characteristics of the patients that develop recurrences. If a recurrence can be curatively treated, survival is not compromised. This is often the case if the recurrence develops in the remaining ovary. We found 34.3% isolated recurrences in the contralateral ovary. In extra-ovarian recurrences, it is disputable whether the FSS caused the recurrence or that the metastatic spread had already occurred and would not have been prevented by the removal of the uterus and contralateral ovary. This is then merely a reflection of the biological behavior than the surgical technique, which is confirmed by our findings that isolated recurrences occurred more in low-risk subtypes and extra-ovarian recurrences more in high-risk subtypes (grade 3, stage IC, clear cell carcinoma). If staging is inadequate, stage III disease can be missed and lead to recurrent disease. In our group, 70% of patients underwent staging with at least omentectomy, peritoneal biopsies, and washings. In 56% of patients, lymph node sampling was performed. Adjuvant chemotherapy was started in almost 51% of patients. Therefore, it is not the FSS itself that is evaluated, but the fertility-sparing treatment as a whole. Despite these limitations, our review includes almost 4000 ovarian cancer patients undergoing FSS, and with the current selection of patients, the procedure seems oncologically safe in low-grade disease. Still, careful consideration remains important, because also after FSS in ovarian cancer, only 44.2% of the patients have a pregnancy wish and 29.5% achieve a pregnancy.
Early stage (endometrioid) endometrial cancer has an excellent prognosis after treatment with hysterectomy and bilateral salpingo-oophorectomy. Hormonal therapy is less effective compared to definitive surgery, although the response rate is 80% in this review. This rate is comparable to previous reviews of Gunderson et al. [15] and Gallos et al. [176], who reported a response rate of 74.6% and 76.2%, respectively. In most studies, patients with complex hyperplasia are included besides endometrial cancer, which will give a more optimistic response rate and therefore, differ from the current analysis. Although hormonal therapy can be an acceptable alternative in terms of initial response, recurrence rate is very high, reaching almost 35%. Therefore, it is important that patients pursue pregnancy soon after remission and that hysterectomy is performed after completion of childbearing [177]. There is no consensus regarding optimal treatment strategy, because different types of progestin, dosage, and administration are used. The chance of complete remission with LNG-IUD is not significantly different compared to oral agents. However, the risk of recurrence is higher after oral administration (45.7% versus 9.5% after LNG-IUD). A possible explanation for this difference is that in two of the 3 studies investigating LNG-IUD, a hysteroscopic resection was also performed, which was not standard in patients who started with oral treatment. In previous studies, this procedure increased the response rate and reduced the chance of recurrence [178]. Most studies are single arm studies which obstructs a proper comparison. It is unknown if a combination of LNG-IUD and oral agents further improves outcome. Metformin can be added to the conservative treatment of endometrial cancer. Women most likely to benefit from this strategy are women with obesity, polycystic ovary syndrome, and insulin resistance. It has been previously reported that obese patients with endometrioid endometrial cancer have less risk of cancer recurrence on metformin [179] and for patients with endometrial cancer, the use of metformin was associated with improved RFS and OS [180]. However, groups are heterogeneous (often also including hyperplasia) and the only available RCT does not show a benefit from the addition of metformin [167].
The treatment of endometrial cancer is rapidly changing as molecular markers are being integrated in the (adjuvant) treatment as they predict the prognosis of patients. In FSS, these markers are not evaluated yet, although they may predict response to conservative treatment. Patients with MMRd tumors may be less responsive than with POLE mutation. Thus far, numbers are too small to draw any conclusions.
We found a relatively high number of fetal losses (31.3%) after FSS for endometrial cancer, but the low number of studies describing obstetric outcome may influence this number. The live birth rate of 72% is comparable to other studies [181].
5. Conclusions
From the above, it becomes clear that counseling by experts is required to help patients making the life-changing decision whether or not to undergo FSS. However, it is difficult to become an expert, as the situation is rare. According to the WHO, a disease is rare if the number of affected people is less than 5 per 10,000. The care for patients with rare diseases is challenged by the small number of patients, lack of validated treatment options, scattering of patients across a country, and limited clinical expertise. Although cervical cancer, ovarian cancer, and endometrial cancer do not meet the official criteria of a rare disease, the care for AYAs with gynecological cancer and a wish to preserve fertility is a unique situation encountering the same problems described for rare diseases. Tailor-made medicine in oncological care is a rapidly growing approach where, based on malignancy-specific characteristics, individualized treatment is given. Patient-specific characteristics are equally important to consider for a specific treatment. AYAs have their unique social situations, personal beliefs, and preferences. The outcome of a process of shared decision-making is important for tailor-made care. Decisions on fertility are not only difficult for the patient, but also for the treating gynecologist. Therefore, management of these patients should involve a (experienced) multidisciplinary team. It is generally accepted that exposure of a medical specialist to a rare health problem or situation is related to knowledge and quality of care. Participation in clinical trials or registration studies is indispensable, as this is the only way to evaluate and improve quality of care. International collaboration will facilitate achieving larger patient numbers and development of guidelines.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/5/1008/s1, Table S1: Search string, Table S2: Newcastle–Ottawa quality assessment scale of included studies on cervical cancer, Table S3: Newcastle–Ottawa quality assessment scale of included studies on epithelial ovarian cancer, Table S4: Newcastle–Ottawa quality assessment scale of included studies on endometrial cancer, Table S5: Literature review of LLETZ, conization or simple trachelectomy in patients with cervical cancer, Table S6: Literature review of vaginal radical trachelectomy in patients with cervical cancer, Table S7: Literature review of abdominal radical trachelectomy in patients with cervical cancer, Table S8: Literature review of FSS versus non-FSS in patients with cervical cancer, Table S9: Literature review of FSS in patients with epithelial ovarian cancer, Table S10: Literature review of FSS versus non-FSS in patients with epithelial ovarian cancer, Table S11: Literature review of FSS and hormonal therapy in patients with endometrial cancer.
Author Contributions
Conceptualization, N.v.T. and C.L.; methodology, W.S. and T.S.; formal analysis, T.S., S.Z. and S.S.; data curation, T.S. and S.S.; writing—original draft preparation, T.S. and S.S.; writing—review and editing, S.Z., F.A., N.v.T. and C.L.; supervision, N.v.T. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization, International Agency for Research on Cancer (IARC), Global Cancer Observatory (GCO) [(accessed on 17 November 2020)]; Available online: https://gco.iarc.fr.
- 2.The Netherlands Cancer Registry, the Netherlands Comprehensive Cancer Organisation (IKNL) [(accessed on 17 November 2020)]; Available online: https://www.iknl.nl/netherlands-cancer-registry.
- 3.Moser E.C., Meunier F. Cancer survivorship: A positive side-effect of more successful cancer treatment. EJC Suppl. 2014;12:1–4. doi: 10.1016/j.ejcsup.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffy C., Allen S. Medical and psychosocial aspects of fertility after cancer. Cancer J. 2009;15:27–33. doi: 10.1097/PPO.0b013e3181976602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prodromidou A., Iavazzo C., Fotiou A., Psomiadou V., Douligeris A., Vorgias G., Kalinoglou N. Short- and long term outcomes after abdominal radical trachelectomy versus radical hysterectomy for early stage cervical cancer: A systematic review of the literature and meta-analysis. Arch. Gynecol. Obstet. 2019;300:25–31. doi: 10.1007/s00404-019-05176-y. [DOI] [PubMed] [Google Scholar]
- 6.Bentivegna E., Maulard A., Pautier P., Chargari C., Gouy S., Morice P. Fertility results and pregnancy outcomes after conservative treatment of cervical cancer: A systematic review of the literature. Fertil. Steril. 2016;106:1195–1211.e5. doi: 10.1016/j.fertnstert.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Plante M. Bulky Early-Stage Cervical Cancer (2-4 cm Lesions): Upfront Radical Trachelectomy or Neoadjuvant Chemotherapy Followed by Fertility-Preserving Surgery: Which Is the Best Option? Int. J. Gynecol. Cancer. 2015;25:722–728. doi: 10.1097/IGC.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 8.Cao D.Y., Yang J.X., Wu X.H., Chen Y.L., Li L., Liu K.J., Cui M.H., Xie X., Wu Y.M., Kong B.H., et al. Comparisons of vaginal and abdominal radical trachelectomy for early-stage cervical cancer: Preliminary results of a multi-center research in China. Br. J. Cancer. 2013;109:2778–2782. doi: 10.1038/bjc.2013.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laios A., Kasius J., Tranoulis A., Gryparis A., Ind T. Obstetric Outcomes in Women With Early Bulky Cervical Cancer Downstaged by Neoadjuvant Chemotherapy to Allow for Fertility-Sparing Surgery: A Meta-analysis and Metaregression. Int. J. Gynecol. Cancer. 2018;28:794–801. doi: 10.1097/IGC.0000000000001232. [DOI] [PubMed] [Google Scholar]
- 10.Plante M., van Trommel N., Lheureux S., Oza A.M., Wang L., Sikorska K., Ferguson S.E., Han K., Amant F. FIGO 2018 stage IB2 (2-4 cm) Cervical cancer treated with Neo-adjuvant chemotherapy followed by fertility Sparing Surgery (CONTESSA); Neo-Adjuvant Chemotherapy and Conservative Surgery in Cervical Cancer to Preserve Fertility (NEOCON-F). A PMHC, DGOG, GCIG/CCRN and multicenter study. Int. J. Gynecol. Cancer. 2019;29:969–975. doi: 10.1136/ijgc-2019-000398. [DOI] [PubMed] [Google Scholar]
- 11.Epithelial Ovarian Cancer Dutch National Guideline, Version 2.3. [(accessed on 17 November 2020)]; Available online: www.oncoline.nl.
- 12.Bentivegna E., Gouy S., Maulard A., Pautier P., Leary A., Colombo N., Morice P. Fertility-sparing surgery in epithelial ovarian cancer: A systematic review of oncological issues. Ann. Oncol. 2016;27:1994–2004. doi: 10.1093/annonc/mdw311. [DOI] [PubMed] [Google Scholar]
- 13.Liu D.C.J., Gao A., Wang Z., Cai L. Fertility sparing surgery vs radical surgery for epithelial ovarian cancer: A meta-analysis of overall survival and disease-free survival. BMC Cancer. 2020;20:320. doi: 10.1186/s12885-020-06828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J.Y., Kim D.Y., Kim J.H., Kim Y.M., Kim K.R., Kim Y.T., Seong S.J., Kim T.J., Kim J.W., Kim S.M., et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002) Eur. J. Cancer. 2013;49:868–874. doi: 10.1016/j.ejca.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Gunderson C.C., Fader A.N., Carson K.A., Bristow R.E. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: A systematic review. Gynecol. Oncol. 2012;125:477–482. doi: 10.1016/j.ygyno.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Frumovitz M., Gershenson D.M. Fertility-sparing therapy for young women with endometrial cancer. Expert Rev. Anticancer Ther. 2006;6:27–32. doi: 10.1586/14737140.6.1.27. [DOI] [PubMed] [Google Scholar]
- 17.Wells G.A., O’Connell B.S.D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 7 November 2020)]; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 18.Abu-Rustum N.R., Sonoda Y. Fertility-sparing surgery in early-stage cervical cancer: Indications and applications. J. Natl. Compr. Cancer Netw. 2010;8:1435–1438. doi: 10.6004/jnccn.2010.0107. [DOI] [PubMed] [Google Scholar]
- 19.Ayhan A., Tohma Y.A., Sahin H., Kocaman E., Tunc M., Haberal A.N. Oncological and obstetric outcomes after fertility-sparing radical abdominal trachelectomy for early stage cervical cancer: A tertiary centre’s 10 years’ experience. J. Obstet. Gynaecol. 2019;39:248–252. doi: 10.1080/01443615.2018.1498830. [DOI] [PubMed] [Google Scholar]
- 20.Balaya V., Lecuru F., Magaud L., Ngo C., Huchon C., Bats A.S., Mathevet P. Perioperative morbidity of radical trachelectomy with lymphadenectomy in early-stage cervical cancer: A French prospective multicentric cohort. J. Gynecol. Oncol. 2019;30:e34. doi: 10.3802/jgo.2019.30.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beiner M.E., Hauspy J., Rosen B., Murphy J., Laframboise S., Nofech-Mozes S., Ismiil N., Rasty G., Khalifa M.A., Covens A. Radical vaginal trachelectomy vs. radical hysterectomy for small early stage cervical cancer: A matched case-control study. Gynecol. Oncol. 2008;110:168–171. doi: 10.1016/j.ygyno.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Bekkers R.L., Keyser K.G., Bulten J., Hanselaar A.G., Schijf C.P., Boonstra H., Massuger L.F. The value of loop electrosurgical conization in the treatment of stage IA1 microinvasive carcinoma of the uterine cervix. Int. J. Gynecol. Cancer. 2002;12:485–489. doi: 10.1136/ijgc-00009577-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Bernardini M., Barrett J., Seaward G., Covens A. Pregnancy outcomes in patients after radical trachelectomy. Am. J. Obstet. Gynecol. 2003;189:1378–1382. doi: 10.1067/S0002-9378(03)00776-2. [DOI] [PubMed] [Google Scholar]
- 24.Biliatis I.K.A., Patel A., Ratnavelu N., Cross P., Chattopadhyay S., Galaal K., Naik R. Small volume stage 1B1 cervical cancer: Is radical surgery still necessary? Gynecol. Oncol. 2012;126:73–77. doi: 10.1016/j.ygyno.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 25.Bisseling K.C., Bekkers R.L., Rome R.M., Quinn M.A. Treatment of microinvasive adenocarcinoma of the uterine cervix: A retrospective study and review of the literature. Gynecol. Oncol. 2007;107:424–430. doi: 10.1016/j.ygyno.2007.07.062. [DOI] [PubMed] [Google Scholar]
- 26.Bogani G., Chiappa V., Vinti D., Somigliana E., Filippi F., Murru G., Murgia F., Martinelli F., Ditto A., Raspagliesi F. Long-term results of fertility-sparing treatment for early-stage cervical cancer. Gynecol. Oncol. 2019;154:89–94. doi: 10.1016/j.ygyno.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Bratila E., Bratila C.P., Coroleuca C.B. Radical Vaginal Trachelectomy with Laparoscopic Pelvic Lymphadenectomy for Fertility Preservation in Young Women with Early-Stage Cervical Cancer. Indian J. Surg. 2016;78:265–270. doi: 10.1007/s12262-015-1351-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capilna M.E., Ioanid N., Scripcariu V., Gavrilescu M.M., Szabo B. Abdominal radical trachelectomy: A Romanian series. Int. J. Gynecol. Cancer. 2014;24:615–619. doi: 10.1097/IGC.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chernyshova A., Kolomiets L., Chekalkin T., Chernov V., Sinilkin I., Gunther V., Marchenko E., Baigonakova G., Kang J.H. Fertility-Sparing Surgery Using Knitted TiNi Mesh Implants and Sentinel Lymph Nodes: A 10-Year Experience. J. Investig. Surg. 2020;13:1–9. doi: 10.1080/08941939.2020.1745965. [DOI] [PubMed] [Google Scholar]
- 30.Choi M.C., Jung S.G., Park H., Lee S.Y., Lee C., Hwang Y.Y., Kim S.J. Fertility preservation by photodynamic therapy combined with conization in young patients with early stage cervical cancer: A pilot study. Photodiagnosis Photodyn. Ther. 2014;11:420–425. doi: 10.1016/j.pdpdt.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Cui R.R., Chen L., Tergas A.I., Hou J.Y., St Clair C.M., Neugut A.I., Ananth C.V., Hershman D.L., Wright J.D. Trends in Use and Survival Associated With Fertility-Sparing Trachelectomy for Young Women With Early-Stage Cervical Cancer. Obstet. Gynecol. 2018;131:1085–1094. doi: 10.1097/AOG.0000000000002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dargent D., Martin X., Sacchetoni A., Mathevet P. Laparoscopic vaginal radical trachelectomy: A treatment to preserve the fertility of cervical carcinoma patients. Cancer. 2000;88:1877–1882. doi: 10.1002/(SICI)1097-0142(20000415)88:8<1877::AID-CNCR17>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 33.Deng X., Zhang Y., Li D., Zhang X., Guo H., Wang F., Sheng X. Abdominal radical trachelectomy guided by sentinel lymph node biopsy for stage IB1 cervical cancer with tumors >2 cm. Oncotarget. 2017;8:3422–3429. doi: 10.18632/oncotarget.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz J.P., Sonoda Y., Leitao M.M., Zivanovic O., Brown C.L., Chi D.S., Barakat R.R., Abu-Rustum N.R. Oncologic outcome of fertility-sparing radical trachelectomy versus radical hysterectomy for stage IB1 cervical carcinoma. Gynecol. Oncol. 2008;111:255–260. doi: 10.1016/j.ygyno.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Du X.L., Sheng X.G., Jiang T., Li Q.S., Yu H., Pan C.X., Lu C.H., Wang C., Song Q.Q. Sentinel lymph node biopsy as guidance for radical trachelectomy in young patients with early stage cervical cancer. BMC Cancer. 2011;11:157. doi: 10.1186/1471-2407-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebisawa K., Takano M., Fukuda M., Fujiwara K., Hada T., Ota Y., Kurotsuchi S., Kanao H., Andou M. Obstetric outcomes of patients undergoing total laparoscopic radical trachelectomy for early stage cervical cancer. Gynecol. Oncol. 2013;131:83–86. doi: 10.1016/j.ygyno.2013.07.108. [DOI] [PubMed] [Google Scholar]
- 37.Fanfani F., Landoni F., Gagliardi M.L., Fagotti A., Preti E., Moruzzi M.C., Monterossi G., Scambia G. Sexual and Reproductive Outcomes in Early Stage Cervical Cancer Patients after Excisional Cone as a Fertility-sparing Surgery: An Italian Experience. J. Reprod. Infertil. 2014;15:29–34. [PMC free article] [PubMed] [Google Scholar]
- 38.Van Gent M.D., van den Haak L.W., Gaarenstroom K.N., Peters A.A., van Poelgeest M.I., Trimbos J.B., de Kroon C.D. Nerve-sparing radical abdominal trachelectomy versus nerve-sparing radical hysterectomy in early-stage (FIGO IA2-IB) cervical cancer: A comparative study on feasibility and outcome. Int. J. Gynecol. Cancer. 2014;24:735–743. doi: 10.1097/IGC.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 39.Gil-Ibanez B., Glickman A., Del Pino M., Boada D., Fuste P., Diaz-Feijoo B., Pahisa J., Torne A. Vaginal fertility-sparing surgery and laparoscopic sentinel lymph node detection in early cervical cancer. Retrospective study with 15 years of follow-up. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;251:23–27. doi: 10.1016/j.ejogrb.2020.05.039. [DOI] [PubMed] [Google Scholar]
- 40.Guo J., Zhang Y., Chen X., Sun L., Chen K., Sheng X. Surgical and Oncologic Outcomes of Radical Abdominal Trachelectomy Versus Hysterectomy for Stage IA2-IB1 Cervical Cancer. J. Minim Invasive Gynecol. 2019;26:484–491. doi: 10.1016/j.jmig.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Hauerberg L., Hogdall C., Loft A., Ottosen C., Bjoern S.F., Mosgaard B.J., Nedergaard L., Lajer H. Vaginal Radical Trachelectomy for early stage cervical cancer. Results of the Danish National Single Center Strategy. Gynecol. Oncol. 2015;138:304–310. doi: 10.1016/j.ygyno.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 42.He Y., Wu Y.M., Zhao Q., Wang T., Wang Y., Kong W.M., Song F., Duan W., Zhu L., Zhang W.Y. Clinical value of cold knife conization as conservative management in patients with microinvasive cervical squamous cell cancer (stage IA1) Int. J. Gynecol. Cancer. 2014;24:1306–1311. doi: 10.1097/IGC.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 43.Helpman L., Grisaru D., Covens A. Early adenocarcinoma of the cervix: Is radical vaginal trachelectomy safe? Gynecol. Oncol. 2011;123:95–98. doi: 10.1016/j.ygyno.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 44.Hertel H., Kohler C., Grund D., Hillemanns P., Possover M., Michels W., Schneider A., German Association of Gynecologic O. Radical vaginal trachelectomy (RVT) combined with laparoscopic pelvic lymphadenectomy: Prospective multicenter study of 100 patients with early cervical cancer. Gynecol. Oncol. 2006;103:506–511. doi: 10.1016/j.ygyno.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 45.Johansen G., Lonnerfors C., Falconer H., Persson J. Reproductive and oncologic outcome following robot-assisted laparoscopic radical trachelectomy for early stage cervical cancer. Gynecol. Oncol. 2016;141:160–165. doi: 10.1016/j.ygyno.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 46.Kasuga Y., Nishio H., Miyakoshi K., Sato S., Sugiyama J., Matsumoto T., Tanaka K., Ochiai D., Minegishi K., Hamatani T., et al. Pregnancy Outcomes After Abdominal Radical Trachelectomy for Early-Stage Cervical Cancer: A 13-Year Experience in a Single Tertiary-Care Center. Int. J. Gynecol. Cancer. 2016;26:163–168. doi: 10.1097/IGC.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 47.Kim J.H., Park J.Y., Kim D.Y., Kim Y.M., Kim Y.T., Nam J.H. Fertility-sparing laparoscopic radical trachelectomy for young women with early stage cervical cancer. BJOG. 2010;117:340–347. doi: 10.1111/j.1471-0528.2009.02446.x. [DOI] [PubMed] [Google Scholar]
- 48.Kim H.S., Choi C.H., Lim M.C., Chang S.J., Kim Y.B., Kim M.A., Kim T.J., Park S.Y., Kim B.G., Song Y.S., et al. Safe criteria for less radical trachelectomy in patients with early-stage cervical cancer: A multicenter clinicopathologic study. Ann. Surg. Oncol. 2012;19:1973–1979. doi: 10.1245/s10434-011-2148-7. [DOI] [PubMed] [Google Scholar]
- 49.Kim M., Ishioka S., Endo T., Baba T., Mizuuchi M., Takada S., Saito T. Possibility of less radical treatment for patients with early invasive uterine cervical cancer. J. Obstet. Gynaecol. Res. 2016;42:876–882. doi: 10.1111/jog.12980. [DOI] [PubMed] [Google Scholar]
- 50.Kim C.H., Abu-Rustum N.R., Chi D.S., Gardner G.J., Leitao M.M., Jr., Carter J., Barakat R.R., Sonoda Y. Reproductive outcomes of patients undergoing radical trachelectomy for early-stage cervical cancer. Gynecol. Oncol. 2012;125:585–588. doi: 10.1016/j.ygyno.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Lanowska M., Mangler M., Speiser D., Bockholdt C., Schneider A., Kohler C., Vasiljeva J., Al-Hakeem M., Vercellino G.F. Radical vaginal trachelectomy after laparoscopic staging and neoadjuvant chemotherapy in women with early-stage cervical cancer over 2 cm: Oncologic, fertility, and neonatal outcome in a series of 20 patients. Int. J. Gynecol. Cancer. 2014;24:586–593. doi: 10.1097/IGC.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 52.Lee S.J., Kim W.Y., Lee J.W., Kim H.S., Choi Y.L., Ahn G.H., Lee J.H., Kim B.G., Bae D.S. Conization using electrosurgical conization and cold coagulation for international federation of gynecology and obstetrics stage IA1 squamous cell carcinomas of the uterine cervix. Int. J. Gynecol. Cancer. 2009;19:407–411. doi: 10.1111/IGC.0b013e3181a1a297. [DOI] [PubMed] [Google Scholar]
- 53.Lee S.W., Kim Y.M., Son W.S., You H.J., Kim D.Y., Kim J.H., Kim Y.T., Nam J.H. The efficacy of conservative management after conization in patients with stage IA1 microinvasive cervical carcinoma. Acta Obstet. Gynecol. Scand. 2009;88:209–215. doi: 10.1080/00016340802596009. [DOI] [PubMed] [Google Scholar]
- 54.Lee C.Y., Chen Y.L., Chiang Y.C., Cheng C.Y., Lai Y.L., Tai Y.J., Hsu H.C., Hwa H.L., Cheng W.F. Outcome and Subsequent Pregnancy after Fertility-Sparing Surgery of Early-Stage Cervical Cancers. Int. J. Environ. Res. Public Health. 2020;17:7103. doi: 10.3390/ijerph17197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J., Li Z., Wang H., Zang R., Zhou Y., Ju X., Ke G., Wu X. Radical abdominal trachelectomy for cervical malignancies: Surgical, oncological and fertility outcomes in 62 patients. Gynecol. Oncol. 2011;121:565–570. doi: 10.1016/j.ygyno.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 56.Li X., Li J., Jiang Z., Xia L., Ju X., Chen X., Wu X. Oncological results and recurrent risk factors following abdominal radical trachelectomy: An updated series of 333 patients. BJOG. 2019;126:1169–1174. doi: 10.1111/1471-0528.15621. [DOI] [PubMed] [Google Scholar]
- 57.Li X., Xia L., Chen X., Fu Y., Wu X. Simple conization and pelvic lymphadenectomy in early-stage cervical cancer: A retrospective analysis and review of the literature. Gynecol. Oncol. 2020;158:231–235. doi: 10.1016/j.ygyno.2020.05.035. [DOI] [PubMed] [Google Scholar]
- 58.Lindsay R.B.K., Shanbhag S., Tolhurst J., Millan D., Siddiqui N. Fertility conserving management of early cervical cancer: Our experience of LLETZ and pelvic lymph node dissection. Int. J. Gynecol. Cancer. 2014;24:118–123. doi: 10.1097/IGC.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 59.Lintner B., Saso S., Tarnai L., Novak Z., Palfalvi L., Del Priore G., Smith J.R., Ungar L. Use of abdominal radical trachelectomy to treat cervical cancer greater than 2 cm in diameter. Int. J. Gynecol. Cancer. 2013;23:1065–1070. doi: 10.1097/IGC.0b013e318295fb41. [DOI] [PubMed] [Google Scholar]
- 60.Lu Q., Zhang Z., Xiao M., Liu C., Zhang Z. The Surgical Morbidity and Oncological Outcome of Total Laparoscopic Radical Trachelectomy Versus Total Laparoscopic Radical Hysterectomy For Early Stage Cervical Cancer: A Retrospective Study With 11-Year Follow-Up. Onco. Targets Ther. 2019;12:7941–7947. doi: 10.2147/OTT.S224525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma L.K., Cao D.Y., Yang J.X., Liu J.T., Shen K., Lang J.H. Pregnancy outcome and obstetric management after vaginal radical trachelectomy. Eur. Rev. Med. Pharmacol. Sci. 2014;18:3019–3024. [PubMed] [Google Scholar]
- 62.Machida H., Iwata T., Okugawa K., Matsuo K., Saito T., Tanaka K., Morishige K., Kobayashi H., Yoshino K., Tokunaga H., et al. Fertility-sparing trachelectomy for early-stage cervical cancer: A proposal of an ideal candidate. Gynecol. Oncol. 2020;156:341–348. doi: 10.1016/j.ygyno.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Machida H., Mandelbaum R.S., Mikami M., Enomoto T., Sonoda Y., Grubbs B.H., Paulson R.J., Roman L.D., Wright J.D., Matsuo K. Characteristics and outcomes of reproductive-aged women with early-stage cervical cancer: Trachelectomy vs hysterectomy. Am. J. Obstet. Gynecol. 2018;219:461.e1–461.e18. doi: 10.1016/j.ajog.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malmsten C., Hellberg P., Bergmark K., Dahm-Kahler P. Long-term fertility, oncological, and quality-of-life outcomes after trachelectomy in early stage cervical cancer. Arch. Gynecol. Obstet. 2019;299:1033–1041. doi: 10.1007/s00404-018-4972-5. [DOI] [PubMed] [Google Scholar]
- 65.Maneo A., Sideri M., Scambia G., Boveri S., Dell’anna T., Villa M., Parma G., Fagotti A., Fanfani F., Landoni F. Simple conization and lymphadenectomy for the conservative treatment of stage IB1 cervical cancer. An Italian experience. Gynecol. Oncol. 2011;123:557–560. doi: 10.1016/j.ygyno.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 66.Maneo A., Chiari S., Bonazzi C., Mangioni C. Neoadjuvant chemotherapy and conservative surgery for stage IB1 cervical cancer. Gynecol. Oncol. 2008;111:438–443. doi: 10.1016/j.ygyno.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 67.Mangler M., Lanowska M., Kohler C., Vercellino F., Schneider A., Speiser D. Pattern of cancer recurrence in 320 patients after radical vaginal trachelectomy. Int. J. Gynecol. Cancer. 2014;24:130–134. doi: 10.1097/IGC.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 68.Marchiole P., Benchaib M., Buenerd A., Lazlo E., Dargent D., Mathevet P. Oncological safety of laparoscopic-assisted vaginal radical trachelectomy (LARVT or Dargent’s operation): A comparative study with laparoscopic-assisted vaginal radical hysterectomy (LARVH) Gynecol. Oncol. 2007;106:132–141. doi: 10.1016/j.ygyno.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Matsuo K., Chen L., Mandelbaum R.S., Melamed A., Roman L.D., Wright J.D. Trachelectomy for reproductive-aged women with early-stage cervical cancer: Minimally invasive surgery versus laparotomy. Am. J. Obstet. Gynecol. 2019;220:469.e1–469.e13. doi: 10.1016/j.ajog.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsuo K., Machida H., Mandelbaum R.S., Mikami M., Enomoto T., Roman L.D., Wright J.D. Trachelectomy for stage IB1 cervical cancer with tumor size >2 cm: Trends and characteristics in the United States. J Gynecol. Oncol. 2018;29:e85. doi: 10.3802/jgo.2018.29.e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muraji M., Sudo T., Nakagawa E., Ueno S., Wakahashi S., Kanayama S., Yamada T., Yamaguchi S., Fujiwara K., Nishimura R. Type II versus type III fertility-sparing abdominal radical trachelectomy for early-stage cervical cancer: A comparison of feasibility of surgical outcomes. Int. J. Gynecol. Cancer. 2012;22:479–483. doi: 10.1097/IGC.0b013e31823fa7bd. [DOI] [PubMed] [Google Scholar]
- 72.Nakajima T., Kasuga A., Hara-Yamashita A., Ikeda Y., Asai-Sato M., Nakao T., Hayashi C., Takeya C., Adachi K., Tsuruga T., et al. Reconstructed uterine length is critical for the prevention of cervical stenosis following abdominal trachelectomy in cervical cancer patients. J. Obstet. Gynaecol. Res. 2020;46:328–336. doi: 10.1111/jog.14153. [DOI] [PubMed] [Google Scholar]
- 73.Nick A.M., Frumovitz M.M., Soliman P.T., Schmeler K.M., Ramirez P.T. Fertility sparing surgery for treatment of early-stage cervical cancer: Open vs. robotic radical trachelectomy. Gynecol. Oncol. 2012;124:276–280. doi: 10.1016/j.ygyno.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishio H., Fujii T., Sugiyama J., Kuji N., Tanaka M., Hamatani T., Miyakoshi K., Minegishi K., Tsuda H., Iwata T., et al. Reproductive and obstetric outcomes after radical abdominal trachelectomy for early-stage cervical cancer in a series of 31 pregnancies. Hum. Reprod. 2013;28:1793–1798. doi: 10.1093/humrep/det118. [DOI] [PubMed] [Google Scholar]
- 75.Okugawa K., Yahata H., Sonoda K., Kodama K., Yagi H., Ohgami T., Yasunaga M., Onoyama I., Kaneki E., Asanoma K., et al. Evaluation of adjuvant chemotherapy after abdominal trachelectomy for cervical cancer: A single-institution experience. Int. J. Clin. Oncol. 2020;26:216–224. doi: 10.1007/s10147-020-01778-8. [DOI] [PubMed] [Google Scholar]
- 76.Park J.Y., Kim D.Y., Suh D.S., Kim J.H., Kim Y.M., Kim Y.T., Nam J.H. Reproductive outcomes after laparoscopic radical trachelectomy for early-stage cervical cancer. J Gynecol. Oncol. 2014;25:9–13. doi: 10.3802/jgo.2014.25.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park J.Y., Joo W.D., Chang S.J., Kim D.Y., Kim J.H., Kim Y.M., Kim Y.T., Nam J.H. Long-term outcomes after fertility-sparing laparoscopic radical trachelectomy in young women with early-stage cervical cancer: An Asan Gynecologic Cancer Group (AGCG) study. J. Surg. Oncol. 2014;110:252–257. doi: 10.1002/jso.23631. [DOI] [PubMed] [Google Scholar]
- 78.Plante M., Gregoire J., Renaud M.C., Roy M. The vaginal radical trachelectomy: An update of a series of 125 cases and 106 pregnancies. Gynecol. Oncol. 2011;121:290–297. doi: 10.1016/j.ygyno.2010.12.345. [DOI] [PubMed] [Google Scholar]
- 79.Plante M., Renaud M.C., Sebastianelli A., Gregoire J. Simple vaginal trachelectomy in women with early-stage low-risk cervical cancer who wish to preserve fertility: The new standard of care? Int. J. Gynecol. Cancer. 2020;30:981–986. doi: 10.1136/ijgc-2020-001432. [DOI] [PubMed] [Google Scholar]
- 80.Raju S.K., Papadopoulos A.J., Montalto S.A., Coutts M., Culora G., Kodampur M., Mehra G., Devaja O. Fertility-sparing surgery for early cervical cancer-approach to less radical surgery. Int. J. Gynecol. Cancer. 2012;22:311–317. doi: 10.1097/IGC.0b013e3182370f51. [DOI] [PubMed] [Google Scholar]
- 81.Renaud M.C., Plante M., Roy M. Combined laparoscopic and vaginal radical surgery in cervical cancer. Gynecol. Oncol. 2000;79:59–63. doi: 10.1006/gyno.2000.5912. [DOI] [PubMed] [Google Scholar]
- 82.Rendon G.J., Lopez Blanco A., Aragona A., Saadi J.M., Di Guilmi J., Arab Eblen C., Heredia Munoz F., Pareja R. Oncological and obstetrical outcomes after neo-adjuvant chemotherapy followed by fertility-sparing surgery in patients with cervical cancer >/=2 cm. Int. J. Gynecol. Cancer. 2020;16:16. doi: 10.1136/ijgc-2020-002076. [DOI] [PubMed] [Google Scholar]
- 83.Rob L., Pluta M., Strnad P., Hrehorcak M., Chmel R., Skapa P., Robova H. A less radical treatment option to the fertility-sparing radical trachelectomy in patients with stage I cervical cancer. Gynecol. Oncol. 2008;111(Suppl. 2):S116–S120. doi: 10.1016/j.ygyno.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 84.Robova H., Halaska M.J., Pluta M., Skapa P., Matecha J., Lisy J., Rob L. Oncological and pregnancy outcomes after high-dose density neoadjuvant chemotherapy and fertility-sparing surgery in cervical cancer. Gynecol. Oncol. 2014;135:213–216. doi: 10.1016/j.ygyno.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 85.Saadi J., Minig L., Noll F., Saraniti G., Cardenas-Rebollo J.M., Perrotta M. Four Surgical Approaches to Cervical Excision during Laparoscopic Radical Trachelectomy for Early Cervical Cancer. J. Minim Invasive Gynecol. 2017;24:869–875. doi: 10.1016/j.jmig.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 86.Saso S., Ghaem-Maghami S., Chatterjee J., Naji O., Farthing A., Mason P., McIndoe A., Hird V., Ungar L., Del Priore G., et al. Abdominal radical trachelectomy in West London. BJOG. 2012;119:187–193. doi: 10.1111/j.1471-0528.2011.03213.x. [DOI] [PubMed] [Google Scholar]
- 87.Shah J.S., Jooya N.D., Woodard T.L., Ramirez P.T., Fleming N.D., Frumovitz M. Reproductive counseling and pregnancy outcomes after radical trachelectomy for early stage cervical cancer. J Gynecol. Oncol. 2019;30:e45. doi: 10.3802/jgo.2019.30.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shepherd J.H. Challenging dogma: Radical conservation surgery for early stage cervical cancer in order to retain fertility. Ann. R Coll. Surg. Engl. 2009;91:181–187. doi: 10.1308/003588409X392108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shinkai S., Ishioka S., Mariya T., Fujibe Y., Kim M., Someya M., Saito T. Pregnancies after vaginal radical trachelectomy (RT) in patients with early invasive uterine cervical cancer: Results from a single institute. BMC Pregnancy Childbirth. 2020;20:248. doi: 10.1186/s12884-020-02949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Slama J., Cerny A., Dusek L., Fischerova D., Zikan M., Kocian R., Germanova A., Cibula D. Results of less radical fertility-sparing procedures with omitted parametrectomy for cervical cancer: 5years of experience. Gynecol. Oncol. 2016;142:401–404. doi: 10.1016/j.ygyno.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Sonoda Y., Chi D.S., Carter J., Barakat R.R., Abu-Rustum N.R. Initial experience with Dargent’s operation: The radical vaginal trachelectomy. Gynecol. Oncol. 2008;108:214–219. doi: 10.1016/j.ygyno.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 92.Sopracordevole F., Chiossi G., Barbero M., Cristoforoni P., Ghiringhello B., Frega A., Tortolani F., Boselli F., Clemente N., Ciavattini A., et al. Surgical approach and long-term clinical outcome in women with microinvasive cervical cancer. Anticancer Res. 2014;34:4345–4349. [PubMed] [Google Scholar]
- 93.Speiser D., Mangler M., Kohler C., Hasenbein K., Hertel H., Chiantera V., Gottschalk E., Lanowska M. Fertility outcome after radical vaginal trachelectomy: A prospective study of 212 patients. Int. J. Gynecol. Cancer. 2011;21:1635–1639. doi: 10.1097/IGC.0b013e3182230294. [DOI] [PubMed] [Google Scholar]
- 94.Spoozak L., Lewin S.N., Burke W.M., Deutsch I., Sun X., Herzog T.J., Wright J.D. Microinvasive adenocarcinoma of the cervix. Am. J. Obstet. Gynecol. 2012;206:80.e1–80.e6. doi: 10.1016/j.ajog.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 95.Tamauchi S., Kajiyama H., Sakata J., Sekiya R., Suzuki S., Mizuno M., Utsumi F., Niimi K., Kotani T., Shibata K., et al. Oncologic and obstetric outcomes of early stage cervical cancer with abdominal radical trachelectomy: Single-institution experience. J. Obstet. Gynaecol. Res. 2016;42:1796–1801. doi: 10.1111/jog.13100. [DOI] [PubMed] [Google Scholar]
- 96.Testa R., Ramirez P.T., Ferreyra H., Saadi J., Franco G., Goldsman M., Perrotta M. Abdominal radical trachelectomy: A safe and feasible option for fertility preservation in developing countries. J. Low Genit. Tract. Dis. 2013;17:378–384. doi: 10.1097/LGT.0b013e31827cce89. [DOI] [PubMed] [Google Scholar]
- 97.Tokunaga H., Watanabe Y., Niikura H., Nagase S., Toyoshima M., Shiro R., Yokoyama Y., Mizunuma H., Ohta T., Nishiyama H., et al. Outcomes of abdominal radical trachelectomy: Results of a multicenter prospective cohort study in a Tohoku Gynecologic Cancer Unit. Int. J. Clin. Oncol. 2015;20:776–780. doi: 10.1007/s10147-014-0763-6. [DOI] [PubMed] [Google Scholar]
- 98.Tomao F., Maruccio M., Preti E.P., Boveri S., Ricciardi E., Zanagnolo V., Landoni F. Conization in Early Stage Cervical Cancer: Pattern of Recurrence in a 10-Year Single-Institution Experience. Int. J. Gynecol. Cancer. 2017;27:1001–1008. doi: 10.1097/IGC.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 99.Tseng J.H., Aloisi A., Sonoda Y., Gardner G.J., Zivanovic O., Abu-Rustum N.R., Leitao M.M., Jr. Long-Term Oncologic Outcomes of Uterine-Preserving Surgery in Young Women With Stage Ib1 Cervical Cancer. Int. J. Gynecol. Cancer. 2018;28:1350–1359. doi: 10.1097/IGC.0000000000001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Uzan C., Gouy S., Desroque D., Pomel C., Duvillard P., Balleyguier C., Haie-Meder C., Morice P. Analysis of a continuous series of 34 young patients with early-stage cervical cancer selected for a vaginal radical trachelectomy: Should “staging” conization be systematically performed before this procedure? Int. J. Gynecol. Cancer. 2013;23:331–336. doi: 10.1097/IGC.0b013e31827ef759. [DOI] [PubMed] [Google Scholar]
- 101.Vieira M.A., Rendon G.J., Munsell M., Echeverri L., Frumovitz M., Schmeler K.M., Pareja R., Escobar P.F., Reis R.D., Ramirez P.T. Radical trachelectomy in early-stage cervical cancer: A comparison of laparotomy and minimally invasive surgery. Gynecol. Oncol. 2015;138:585–589. doi: 10.1016/j.ygyno.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 102.Wang A., Cui G., Jin C., Wang Y., Tian X. Multicenter research on tumor and pregnancy outcomes in patients with early-stage cervical cancer after fertility-sparing surgery. J. Int. Med. Res. 2019;47:2881–2889. doi: 10.1177/0300060519845974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X., Bi Y., Wu H., Wu M., Li L. Oncologic and obstetric outcomes after conization for adenocarcinoma in situ or stage IA1 cervical cancer. Sci. Rep. 2020;10:19920. doi: 10.1038/s41598-020-75512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wethington S.L., Cibula D., Duska L.R., Garrett L., Kim C.H., Chi D.S., Sonoda Y., Abu-Rustum N.R. An international series on abdominal radical trachelectomy: 101 patients and 28 pregnancies. Int. J. Gynecol. Cancer. 2012;22:1251–1257. doi: 10.1097/IGC.0b013e318263eee2. [DOI] [PubMed] [Google Scholar]
- 105.Wright J.D., Nathavithrana R., Lewin S.N., Sun X., Deutsch I., Burke W.M., Herzog T.J. Fertility-conserving surgery for young women with stage IA1 cervical cancer: Safety and access. Obstet. Gynecol. 2010;115:585–590. doi: 10.1097/AOG.0b013e3181d06b68. [DOI] [PubMed] [Google Scholar]
- 106.Yan H., Liu Z., Fu X., Li Y., Che H., Mo R., Song L. Long-term outcomes of radical vaginal trachelectomy and laparoscopic pelvic lymphadenectomy after neoadjuvant chemotherapy for the IB1 cervical cancer: A series of 60 cases. Int. J. Surg. 2016;29:38–42. doi: 10.1016/j.ijsu.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 107.Yoon A., Choi C.H., Lee Y.Y., Kim T.J., Lee J.W., Kim B.G., Bae D.S. Perioperative Outcomes of Radical Trachelectomy in Early-Stage Cervical Cancer: Vaginal Versus Laparoscopic Approaches. Int. J. Gynecol. Cancer. 2015;25:1051–1057. doi: 10.1097/IGC.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 108.Yoshino A.I., Kobayashi E., Kodama M., Hashimoto K., Ueda Y., Sawada K., Tomimatsu T., Kimura T. Oncological and Reproductive Outcomes of Abdominal Radical Trachelectomy. Anticancer Res. 2020;40:5939–5947. doi: 10.21873/anticanres.14615. [DOI] [PubMed] [Google Scholar]
- 109.Zhang D., Ge H., Li J., Wu X. A new method of surgical margin assuring for abdominal radical trachelectomy in frozen section. Eur. J. Cancer. 2015;51:734–741. doi: 10.1016/j.ejca.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 110.Zhang D., Li J., Ge H., Ju X., Chen X., Tang J., Wu X. Surgical and pathological outcomes of abdominal radical trachelectomy versus hysterectomy for early-stage cervical cancer. Int. J. Gynecol. Cancer. 2014;24:1312–1318. doi: 10.1097/IGC.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 111.Zusterzeel P.L., Pol F.J., van Ham M., Zweemer R.P., Bekkers R.L., Massuger L.F., Verheijen R.H. Vaginal Radical Trachelectomy for Early-Stage Cervical Cancer: Increased Recurrence Risk for Adenocarcinoma. Int. J. Gynecol. Cancer. 2016;26:1293–1299. doi: 10.1097/IGC.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 112.Baek M.H., Park J.Y., Kim D.Y., Suh D.S., Kim J.H., Kim Y.M., Kim Y.T. Feasibility and safety of fertility-sparing surgery in epithelial ovarian cancer with dense adhesion: A long-term result from a single institution. J. Gynecol. Oncol. 2020;31:e85. doi: 10.3802/jgo.2020.31.e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen J., Wang F.F., Zhang Y., Yang B., Ai J.H., Wang X.Y., Cheng X.D., Li K.Z. Oncological and Reproductive Outcomes of Fertility-sparing Surgery in Women with Early-stage Epithelial Ovarian Carcinoma: A Multicenter Retrospective Study. Curr Med. Sci. 2020;40:745–752. doi: 10.1007/s11596-020-2239-4. [DOI] [PubMed] [Google Scholar]
- 114.Colombo N., Parma G., Lapresa M.T., Maggi F., Piantanida P., Maggioni A. Role of conservative surgery in ovarian cancer: The European experience. Int. J. Gynecol. Cancer. 2005;15(Suppl. 3):206–211. doi: 10.1111/j.1525-1438.2005.00428.x. [DOI] [PubMed] [Google Scholar]
- 115.Crafton S.M., Cohn D.E., Llamocca E.N., Louden E., Rhoades J., Felix A.S. Fertility-sparing surgery and survival among reproductive-age women with epithelial ovarian cancer in 2 cancer registries. Cancer. 2020;126:1217–1224. doi: 10.1002/cncr.32620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ditto A., Martinelli F., Bogani G., Lorusso D., Carcangiu M., Chiappa V., Reato C., Donfrancesco C., De Carrillo K.J., Raspagliesi F. Long-term safety of fertility sparing surgery in early stage ovarian cancer: Comparison to standard radical surgical procedures. Gynecol. Oncol. 2015;138:78–82. doi: 10.1016/j.ygyno.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 117.Fakhr I., Abd-Allah M., Ramzy S., Mohamed A.M., Saber A. Outcome of fertility preserving surgery in early stage ovarian cancer. J. Egypt Natl. Cancer Inst. 2013;25:219–222. doi: 10.1016/j.jnci.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 118.Fruscio R., Ceppi L., Corso S., Galli F., Dell’Anna T., Dell’Orto F., Giuliani D., Garbi A., Chiari S., Mangioni C., et al. Long-term results of fertility-sparing treatment compared with standard radical surgery for early-stage epithelial ovarian cancer. Br. J. Cancer. 2016;115:641–648. doi: 10.1038/bjc.2016.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ghezzi F., Cromi A., Fanfani F., Malzoni M., Ditto A., De Iaco P., Uccella S., Gallotta V., Raspagliesi F., Scambia G. Laparoscopic fertility-sparing surgery for early ovarian epithelial cancer: A multi-institutional experience. Gynecol. Oncol. 2016;141:461–465. doi: 10.1016/j.ygyno.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 120.Gouy S., Saidani M., Maulard A., Bach-Hamba S., Bentivegna E., Leary A., Pautier P., Devouassoux-Shisheboran M., Genestie C., Morice P. Results of Fertility-Sparing Surgery for Expansile and Infiltrative Mucinous Ovarian Cancers. Oncologist. 2018;23:324–327. doi: 10.1634/theoncologist.2017-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu J., Zhu L.R., Liang Z.Q., Meng Y.G., Guo H.Y., Qu P.P., Ma C.L., Xu C.J., Yuan B.B. Clinical outcomes of fertility-sparing treatments in young patients with epithelial ovarian carcinoma. J. Zhejiang Univ. Sci. B. 2011;12:787–795. doi: 10.1631/jzus.B1100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang X., Yang J., Yu M., Xie W., Cao D., Wu M., Pan L., Huang H., You Y., Shen K. Oncofertility in patients with stage I epithelial ovarian cancer: Fertility-sparing surgery in young women of reproductive age. World J. Surg. Oncol. 2017;15:154. doi: 10.1186/s12957-017-1222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johansen G., Dahm-Kahler P., Staf C., Floter Radestad A., Rodriguez-Wallberg K.A. A Swedish Nationwide prospective study of oncological and reproductive outcome following fertility-sparing surgery for treatment of early stage epithelial ovarian cancer in young women. BMC Cancer. 2020;20:1009. doi: 10.1186/s12885-020-07511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kajiyama H., Suzuki S., Yoshikawa N., Kawai M., Mizuno K., Yamamuro O., Nagasaka T., Shibata K., Kikkawa F. Fertility-sparing surgery and oncologic outcome among patients with early-stage ovarian cancer ~propensity score- matched analysis~. BMC Cancer. 2019;19:1235. doi: 10.1186/s12885-019-6432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kwon Y.S., Hahn H.S., Kim T.J., Lee I.H., Lim K.T., Lee K.H., Shim J.U., Mok J.E. Fertility preservation in patients with early epithelial ovarian cancer. J Gynecol. Oncol. 2009;20:44–47. doi: 10.3802/jgo.2009.20.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee J.Y., Jo Y.R., Kim T.H., Kim H.S., Kim M.A., Kim J.W., Park N.H., Song Y.S. Safety of fertility-sparing surgery in primary mucinous carcinoma of the ovary. Cancer Res. Treat. 2015;47:290–297. doi: 10.4143/crt.2014.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morice P., Leblanc E., Rey A., Baron M., Querleu D., Blanchot J., Duvillard P., Lhomme C., Castaigne D., Classe J.M., et al. Gcclcc; Sfog, Conservative treatment in epithelial ovarian cancer: Results of a multicentre study of the GCCLCC (Groupe des Chirurgiens de Centre de Lutte Contre le Cancer) and SFOG (Societe Francaise d’Oncologie Gynecologique) Hum. Reprod. 2005;20:1379–1385. doi: 10.1093/humrep/deh777. [DOI] [PubMed] [Google Scholar]
- 128.Mueller J.J., Lajer H., Mosgaard B.J., Bach Hamba S., Morice P., Gouy S., Hussein Y., Soslow R.A., Schlappe B.A., Zhou Q.C., et al. International Study of Primary Mucinous Ovarian Carcinomas Managed at Tertiary Medical Centers. Int. J. Gynecol. Cancer. 2018;28:915–924. doi: 10.1097/IGC.0000000000001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nasioudis D., Mastroyannis S.A., Haggerty A.F., Giuntoli R.L., 2nd, Morgan M.A., Ko E.M., Latif N.A. Fertility preserving surgery for high-grade epithelial ovarian carcinoma confined to the ovary. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;248:63–70. doi: 10.1016/j.ejogrb.2020.01.039. [DOI] [PubMed] [Google Scholar]
- 130.Park J.Y., Kim D.Y., Suh D.S., Kim J.H., Kim Y.M., Kim Y.T., Nam J.H. Outcomes of fertility-sparing surgery for invasive epithelial ovarian cancer: Oncologic safety and reproductive outcomes. Gynecol. Oncol. 2008;110:345–353. doi: 10.1016/j.ygyno.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 131.Park J.Y., Suh D.S., Kim J.H., Kim Y.M., Kim Y.T., Nam J.H. Outcomes of fertility-sparing surgery among young women with FIGO stage I clear cell carcinoma of the ovary. Int. J. Gynaecol. Obstet. 2016;134:49–52. doi: 10.1016/j.ijgo.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 132.Ratanasrithong P., Benjapibal M. Pregnancy Outcomes after Conservative Surgery for Early-Stage Ovarian Neoplasms. Asian Pac. J. Cancer Prev. 2017;18:2083–2087. doi: 10.22034/APJCP.2017.18.8.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Satoh T., Hatae M., Watanabe Y., Yaegashi N., Ishiko O., Kodama S., Yamaguchi S., Ochiai K., Takano M., Yokota H., et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: A proposal for patient selection. J. Clin. Oncol. 2010;28:1727–1732. doi: 10.1200/JCO.2009.24.8617. [DOI] [PubMed] [Google Scholar]
- 134.Schilder J.M., Thompson A.M., DePriest P.D., Ueland F.R., Cibull M.L., Kryscio R.J., Modesitt S.C., Lu K.H., Geisler J.P., Higgins R.V., et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol. Oncol. 2002;87:1–7. doi: 10.1006/gyno.2002.6805. [DOI] [PubMed] [Google Scholar]
- 135.Schlaerth A.C., Chi D.S., Poynor E.A., Barakat R.R., Brown C.L. Long-term survival after fertility-sparing surgery for epithelial ovarian cancer. Int. J. Gynecol. Cancer. 2009;19:1199–1204. doi: 10.1111/IGC.0b013e31819d82c3. [DOI] [PubMed] [Google Scholar]
- 136.Watanabe T., Soeda S., Nishiyama H., Kiko Y., Tokunaga H., Shigeta S., Yaegashi N., Yamada H., Ohta T., Nagase S., et al. Clinical and reproductive outcomes of fertility-sparing surgery in stage I epithelial ovarian cancer. Mol. Clin. Oncol. 2020;12:44–50. doi: 10.3892/mco.2019.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yin J., Wang Y., Shan Y., Li Y., Jin Y., Pan L. Pregnancy and oncologic outcomes of early stage low grade epithelial ovarian cancer after fertility sparing surgery: A retrospective study in one tertiary hospital of China. J. Ovarian Res. 2019;12:44. doi: 10.1186/s13048-019-0520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoshihara M., Kajiyama H., Tamauchi S., Suzuki S., Takahashi K., Matsui S., Kikkawa F. Prognostic factors and effects of fertility-sparing surgery in women of reproductive age with ovarian clear-cell carcinoma: A propensity score analysis. J. Gynecol. Oncol. 2019;30:e102. doi: 10.3802/jgo.2019.30.e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ayhan A., Tohma Y.A., Tunc M. Fertility preservation in early-stage endometrial cancer and endometrial intraepithelial neoplasia: A single-center experience. Taiwan J. Obstet. Gynecol. 2020;59:415–419. doi: 10.1016/j.tjog.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 140.Casadio P., La Rosa M., Alletto A., Magnarelli G., Arena A., Fontana E., Fabbri M., Giovannico K., Virgilio A., Raimondo D., et al. Fertility Sparing Treatment of Endometrial Cancer with and without Initial Infiltration of Myometrium: A Single Center Experience. Cancers. 2020;12:29. doi: 10.3390/cancers12123571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chae S.H., Shim S.H., Lee S.J., Lee J.Y., Kim S.N., Kang S.B. Pregnancy and oncologic outcomes after fertility-sparing management for early stage endometrioid endometrial cancer. Int. J. Gynecol. Cancer. 2019;29:77–85. doi: 10.1136/ijgc-2018-000036. [DOI] [PubMed] [Google Scholar]
- 142.Chen M., Jin Y., Li Y., Bi Y., Shan Y., Pan L. Oncologic and reproductive outcomes after fertility-sparing management with oral progestin for women with complex endometrial hyperplasia and endometrial cancer. Int. J. Gynaecol. Obstet. 2016;132:34–38. doi: 10.1016/j.ijgo.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 143.Chung Y.S., Woo H.Y., Lee J.Y., Park E., Nam E.J., Kim S., Kim S.W., Kim Y.T. Mismatch repair status influences response to fertility-sparing treatment of endometrial cancer. Am. J. Obstet. Gynecol. 2020;9:9. doi: 10.1016/j.ajog.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 144.Dursun P., Erkanli S., Guzel A.B., Gultekin M., Tarhan N.C., Altundag O., Demirkiran F., Bese T., Yildirim Y., Bozdag G., et al. A Turkish Gynecologic Oncology Group study of fertility-sparing treatment for early-stage endometrial cancer. Int. J. Gynaecol. Obstet. 2012;119:270–273. doi: 10.1016/j.ijgo.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 145.Eftekhar Z., Izadi-Mood N., Yarandi F., Shojaei H., Rezaei Z., Mohagheghi S. Efficacy of megestrol acetate (megace) in the treatment of patients with early endometrial adenocarcinoma: Our experiences with 21 patients. Int. J. Gynecol. Cancer. 2009;19:249–252. doi: 10.1111/IGC.0b013e31819c5372. [DOI] [PubMed] [Google Scholar]
- 146.Falcone F., Laurelli G., Losito S., Di Napoli M., Granata V., Greggi S. Fertility preserving treatment with hysteroscopic resection followed by progestin therapy in young women with early endometrial cancer. J. Gynecol. Oncol. 2017;28:e2. doi: 10.3802/jgo.2017.28.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Falcone F., Leone Roberti Maggiore U., Di Donato V., Perrone A.M., Frigerio L., Bifulco G., Polterauer S., Casadio P., Cormio G., Masciullo V., et al. Fertility-sparing treatment for intramucous, moderately differentiated, endometrioid endometrial cancer: A Gynecologic Cancer Inter-Group (GCIG) study. J. Gynecol. Oncol. 2020;31:e74. doi: 10.3802/jgo.2020.31.e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fujiwara H., Jobo T., Takei Y., Saga Y., Imai M., Arai T., Taneichi A., Machida S., Takahashi Y., Suzuki M. Fertility-sparing treatment using medroxyprogesterone acetate for endometrial carcinoma. Oncol. Lett. 2012;3:1002–1006. doi: 10.3892/ol.2012.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Greenwald Z.R., Huang L.N., Wissing M.D., Franco E.L., Gotlieb W.H. Does hormonal therapy for fertility preservation affect the survival of young women with early-stage endometrial cancer? Cancer. 2017;123:1545–1554. doi: 10.1002/cncr.30529. [DOI] [PubMed] [Google Scholar]
- 150.Hahn H.S., Yoon S.G., Hong J.S., Hong S.R., Park S.J., Lim J.Y., Kwon Y.S., Lee I.H., Lim K.T., Lee K.H., et al. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int. J. Gynecol. Cancer. 2009;19:1068–1073. doi: 10.1111/IGC.0b013e3181aae1fb. [DOI] [PubMed] [Google Scholar]
- 151.Harrison R.F., He W., Fu S., Zhao H., Sun C.C., Suidan R.S., Woodard T.L., Rauh-Hain J.A., Westin S.N., Giordano S.H., et al. National patterns of care and fertility outcomes for reproductive-aged women with endometrial cancer or atypical hyperplasia. Am. J. Obstet. Gynecol. 2019;221:474.e1–474.e11. doi: 10.1016/j.ajog.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim M.K., Seong S.J., Kang S.B., Bae D.S., Kim J.W., Nam J.H., Lim M.C., Lee T.S., Kim S., Paek J. Six months response rate of combined oral medroxyprogesterone/levonorgestrel-intrauterine system for early-stage endometrial cancer in young women: A Korean Gynecologic-Oncology Group Study. J. Gynecol. Oncol. 2019;30:e47. doi: 10.3802/jgo.2019.30.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Laurelli G., Falcone F., Gallo M.S., Scala F., Losito S., Granata V., Cascella M., Greggi S. Long-Term Oncologic and Reproductive Outcomes in Young Women With Early Endometrial Cancer Conservatively Treated: A Prospective Study and Literature Update. Int. J. Gynecol. Cancer. 2016;26:1650–1657. doi: 10.1097/IGC.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 154.Mitsuhashi A., Habu Y., Kobayashi T., Kawarai Y., Ishikawa H., Usui H., Shozu M. Long-term outcomes of progestin plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer patients. J. Gynecol. Oncol. 2019;30:e90. doi: 10.3802/jgo.2019.30.e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Park J.Y., Seong S.J., Kim T.J., Kim J.W., Kim S.M., Bae D.S., Nam J.H. Pregnancy outcomes after fertility-sparing management in young women with early endometrial cancer. Obstet. Gynecol. 2013;121:136–142. doi: 10.1097/AOG.0b013e31827a0643. [DOI] [PubMed] [Google Scholar]
- 156.Park J.Y.K.D., Kim T.J., Kim J.W., Kim J.H., Kim Y.M., Kim Y.T., Bae D.S., Nam J.H. Hormonal therapy for women with stage IA endometrial cancer of all grades. Obstet. Gynecol. 2013;122:7–14. doi: 10.1097/AOG.0b013e3182964ce3. [DOI] [PubMed] [Google Scholar]
- 157.Perri T., Korach J., Gotlieb W.H., Beiner M., Meirow D., Friedman E., Ferenczy A., Ben-Baruch G. Prolonged conservative treatment of endometrial cancer patients: More than 1 pregnancy can be achieved. Int. J. Gynecol. Cancer. 2011;21:72–78. doi: 10.1097/IGC.0b013e31820003de. [DOI] [PubMed] [Google Scholar]
- 158.Pronin S.M., Novikova O.V., Andreeva J.Y., Novikova E.G. Fertility-Sparing Treatment of Early Endometrial Cancer and Complex Atypical Hyperplasia in Young Women of Childbearing Potential. Int. J. Gynecol. Cancer. 2015;25:1010–1014. doi: 10.1097/IGC.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 159.Sato M., Arimoto T., Kawana K., Miyamoto Y., Ikeda Y., Tomio K., Tanikawa M., Sone K., Mori-Uchino M., Tsuruga T., et al. Measurement of endometrial thickness by transvaginal ultrasonography to predict pathological response to medroxyprogesterone acetate in patients with grade 1 endometrioid adenocarcinoma. Mol. Clin. Oncol. 2016;4:492–496. doi: 10.3892/mco.2016.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Son J., Carr C., Yao M., Radeva M., Priyadarshini A., Marquard J., Michener C.M., AlHilli M. Endometrial cancer in young women: Prognostic factors and treatment outcomes in women aged ≤40 years. Int. J. Gynecol. Cancer. 2020;30:631–639. doi: 10.1136/ijgc-2019-001105. [DOI] [PubMed] [Google Scholar]
- 161.Ushijima K., Yahata H., Yoshikawa H., Konishi I., Yasugi T., Saito T., Nakanishi T., Sasaki H., Saji F., Iwasaka T., et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J. Clin. Oncol. 2007;25:2798–2803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- 162.Wang C.J., Chao A., Yang L.Y., Hsueh S., Huang Y.T., Chou H.H., Chang T.C., Lai C.H. Fertility-preserving treatment in young women with endometrial adenocarcinoma: A long-term cohort study. Int. J. Gynecol. Cancer. 2014;24:718–728. doi: 10.1097/IGC.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 163.Wheeler D.T., Bristow R.E., Kurman R.J. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am. J. Surg. Pathol. 2007;31:988–998. doi: 10.1097/PAS.0b013e31802d68ce. [DOI] [PubMed] [Google Scholar]
- 164.Xu Z., Tian Y., Fu J., Xu J., Bao D., Wang G. Efficacy and prognosis of fertility-preserved hysteroscopic surgery combined with progesterone in the treatment of complex endometrial hyperplasia and early endometrial carcinoma. J. BUON. 2020;25:1525–1533. [PubMed] [Google Scholar]
- 165.Yamagami W., Susumu N., Makabe T., Sakai K., Nomura H., Kataoka F., Hirasawa A., Banno K., Aoki D. Is repeated high-dose medroxyprogesterone acetate (MPA) therapy permissible for patients with early stage endometrial cancer or atypical endometrial hyperplasia who desire preserving fertility? J. Gynecol. Oncol. 2018;29:e21. doi: 10.3802/jgo.2018.29.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang B., Xu Y., Zhu Q., Xie L., Shan W., Ning C., Xie B., Shi Y., Luo X., Zhang H., et al. Treatment efficiency of comprehensive hysteroscopic evaluation and lesion resection combined with progestin therapy in young women with endometrial atypical hyperplasia and endometrial cancer. Gynecol. Oncol. 2019;153:55–62. doi: 10.1016/j.ygyno.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 167.Yang B.Y., Gulinazi Y., Du Y., Ning C.C., Cheng Y.L., Shan W.W., Luo X.Z., Zhang H.W., Zhu Q., Ma F.H., et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: A randomised controlled trial. BJOG. 2020;127:848–857. doi: 10.1111/1471-0528.16108. [DOI] [PubMed] [Google Scholar]
- 168.Yang Y.F., Liao Y.Y., Liu X.L., Su S.G., Li L.Z., Peng N.F. Prognostic factors of regression and relapse of complex atypical hyperplasia and well-differentiated endometrioid carcinoma with conservative treatment. Gynecol. Oncol. 2015;139:419–423. doi: 10.1016/j.ygyno.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 169.Bhatla N., Berek J.S., Cuello Fredes M., Denny L.A., Grenman S., Karunaratne K., Kehoe S.T., Konishi I., Olawaiye A.B., Prat J., et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019;145:129–135. doi: 10.1002/ijgo.12749. [DOI] [PubMed] [Google Scholar]
- 170.Prat J., Oncology F.C.o.G. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 171.McAlpine J., Leon-Castillo A., Bosse T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J. Pathol. 2018;244:538–549. doi: 10.1002/path.5034. [DOI] [PubMed] [Google Scholar]
- 172.Hulsbosch S., Koskas M., Tomassetti C., De Sutter P., Wildiers H., Neven P., D’Hooghe T., Amant F. A Real-Life Analysis of Reproductive Outcome after Fertility Preservation in Female Cancer Patients. Gynecol. Obstet. Invest. 2018;83:156–163. doi: 10.1159/000478045. [DOI] [PubMed] [Google Scholar]
- 173.Taarnhoj G.A., Christensen I.J., Lajer H., Fuglsang K., Jeppesen M.M., Kahr H.S., Hogdall C. Risk of recurrence, prognosis, and follow-up for Danish women with cervical cancer in 2005-2013: A national cohort study. Cancer. 2018;124:943–951. doi: 10.1002/cncr.31165. [DOI] [PubMed] [Google Scholar]
- 174.Yamagami W.A.D. Annual report of the Committee on Gynecologic Oncology, the Japan Society of Obstetrics and Gynecology. J. Obstet. Gynaecol. Res. 2015;41:167–177. doi: 10.1111/jog.12596. [DOI] [PubMed] [Google Scholar]
- 175.Cibula D.P.R., Planchamp F., Avall-Lundqvist E., Fischerova D., Haie Meder C., Köhler C., Landoni F., Lax S., Lindegaard J.C., Mahantshetty U., et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients with Cervical Cancer. Int. J. Gynecol. Cancer. 2018;28:641–655. doi: 10.1097/IGC.0000000000001216. [DOI] [PubMed] [Google Scholar]
- 176.Gallos I.D., Yap J., Rajkhowa M., Luesley D.M., Coomarasamy A., Gupta J.K. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2012;207:266.e1–266.e12. doi: 10.1016/j.ajog.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 177.Colombo N., Amant F., Bosse T., González-Martín A., Ledermann J., Marth C., Nout R., Querleu D., Mirza M.R., Sessa C. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016;27:16–41. doi: 10.1093/annonc/mdv484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fan Z., Li H., Hu R., Liu Y., Liu X., Gu L. Fertility-Preserving Treatment in Young Women With Grade 1 Presumed Stage IA Endometrial Adenocarcinoma: A Meta-Analysis. Int. J. Gynecol. Cancer. 2018;28:385–393. doi: 10.1097/IGC.0000000000001164. [DOI] [PubMed] [Google Scholar]
- 179.Hall C.S.R., Gehlot A., Zorn K.K., Burnett A.F. Use of Metformin in Obese Women With Type I Endometrial Cancer Is Associated With a Reduced Incidence of Cancer Recurrence. Int. J. Gynecol. Cancer. 2016;26:313–317. doi: 10.1097/IGC.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 180.Ko E.M.W.P., Jackson A., Clark L., Franasiak J., Bolac C., Havrilesky L.J., Secord A.A., Moore D.T., Gehrig P.A., Bae-Jump V. Metformin is associated with improved survival in endometrial cancer. Gynecol. Oncol. 2014;132:438–442. doi: 10.1016/j.ygyno.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 181.Gerstl B., Sullivan E., Vallejo M., Koch J., Johnson M., Wand H., Webber K., Ives A., Anazodo A. Reproductive outcomes following treatment for a gynecological cancer diagnosis: A systematic review. J. Cancer Sur. 2019;13:269–281. doi: 10.1007/s11764-019-00749-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.