Abstract
Summary: A 12-year-old female patient presented with unilateral sensorineural hearing loss. Distortion-product otoacoustic emission testing failed to reveal any measurable emissions in the affected side. MR imaging did not reveal labyrinthine malformation. Three-dimensional Fourier transformation–constructive interference in steady-state MR images showed a thin cochlear branch. We speculated that mumps infection or developmental malformation caused the unilateral sensorineural hearing loss.
Recent development of high-resolution T2-weighted MR imaging has enabled the delineation of detailed anatomic structures in the internal auditory canal (IAC), with good contrast between CSF and nerves. The facial nerve and the superior vestibular, inferior vestibular, and cochlear branches of the vestibulocochlear nerve (VCN) can be identified in the lateral portion of the IAC, particularly on the cross-sectional images of the nerves (1, 2). We report a case of unilateral sensorineural hearing loss (SNHL) occurring in a patient with a history of mumps, who had a thin cochlear branch shown on the MR images.
Case Report
A 12-year-old female patient presented at our institute with unilateral hearing impairment. She had contracted mumps at the age of 3 years. A survey for the deaf at age 6 years revealed hearing loss on the left side. Nonetheless, no further examination or treatment was performed at that time.
An audiogram and auditory brain stem response confirmed SNHL in the left ear. Hearing in the right ear was normal. Distortion-product otoacoustic emission testing, the level of which is correlated with the degree of damage in the cochlea, failed to show any measurable emissions on the left side consistent with cochlear damage. There was no family history of hearing problems.
MR imaging was performed on a 1.5-T superconductive system (Magnetom Vision, Siemens-Asahi Medical Technologies), including axial 3D Fourier transformation–constructive interference in steady state (3DFT-CISS), T1-weighted spin-echo, and T2-weighted fast spin-echo imaging. The parameters of the CISS sequence were as follows: one 37.8-mm-thick slab; 60 partitions; 12.3/5.9/1 (TR/TE/excitations); matrix, 256 × 512; field of view, 160 mm; and flip angle, 70°. The CISS images were reconstructed perpendicular to the long axis of the IAC by using a built-in multiplanar reconstruction software program.
These images showed a thin cochlear branch in the left IAC. The cochlear branch was the smallest segment of the VCN and was smaller than the facial nerve (Fig 1A and B). On the normal right side, the cochlear branch was the largest segment of the VCN and was larger than the facial nerve (Fig 1C and D). The IAC on the left side seemed to be smaller than that on the right side, based on visual observation, particularly near the fundus. The inner ear structures appeared otherwise normal on the MR images; labyrinthine malformation, including an enlarged endolymphatic duct/sac or brain stem abnormality, was not seen.
FIG 1.
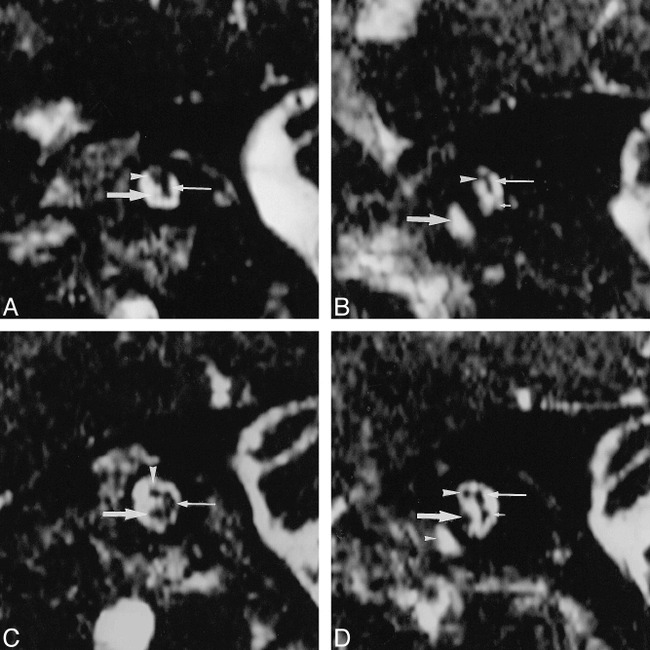
Images from the case of a 12-year-old female patient who presented at our institute with unilateral hearing impairment.
A, Abnormal left ear. Parasagittal 3DFT-CISS (12.3/5.9/1; flip angle, 70°) reconstruction images obtained perpendicular to the IAC at the midportion in the affected left side. The facial nerve is depicted in the superior and anterior position in the IAC (arrowhead). The common vestibular nerve is identified (thin arrow). A thin cochlear branch arises (arrow) from the VCN.
B, Abnormal left ear. Parasagittal 3DFT-CISS reconstruction images obtained perpendicular to the IAC at the lateral portion. The common vestibular nerve (not identified on this image) divides into the superior vestibular branch (thin arrow) and the inferior vestibular branch (short arrow) (poorly visualized). The cochlear branch is not visualized. The IAC seems to be slightly narrow compared with that on the right side (see panel D). The facial nerve is identified (arrowhead). The basal turn of the cochlea is shown (arrow). The vestibular branches also seem to be small in comparison with those on the right side (see panel D). Any vestibular abnormality, however, could be detected in the patient.
C, Normal right ear. Parasagittal 3DFT-CISS (12.3/5.9/1; flip angle, 70°) reconstruction images obtained perpendicular to the IAC at the midportion in the normal right side. The cochlear branch (arrow) is larger than the facial nerve (arrowhead). The common vestibular nerve is shown (thin arrow).
D, Normal right ear. Parasagittal 3DFT-CISS reconstruction images obtained perpendicular to the IAC at the lateral portion. The common vestibular nerve divides into the superior vestibular branch (thin arrow) and the inferior vestibular branch (short arrow). The cochlear branch (arrow) is larger than the facial nerve (arrowhead). Small arrowhead, the cochlea.
Discussion
SNHL is caused by dysfunction of the cochlea, the VCN, or central auditory pathways. In children with SNHL, detection of inner ear malformations is important for understanding the cause of hearing loss. CT and MR imaging are helpful in investigating developmental abnormalities of the inner ear. Although CT performed with bone algorithms could show the osseous labyrinth, 3DFT gradient-echo imaging with multiplanar reconstruction is superior in recognizing membranous labyrinth, particularly the detailed anatomic relationship of the inner ear (ie, abnormalities of the three branches of the VCN) (3).
Kim et al (4) described the relative size of the facial nerve and the branches of the VCN in the lateral portion of the IAC on fast T2-weighted images. In 88% of the 58 cases, the cochlear branch was the largest segment of the VCN and the inferior vestibular branch was the smallest segment. Furthermore, the relative sizes of the four nerves were symmetric in 70% of the 23 participants. Casselman et al (5) reported that the cochlear branch of the VCN was the same size or larger than the facial nerve in 17 of 20 normal inner ears on CISS images. Even in the remaining three inner ears, in which the cochlear branch was slightly smaller than the facial nerve, the diameters of both nerves were nearly the same. In our case, the cochlear branch was the smallest segment of the VCN on the patient's abnormal side. This suggested atrophy or hypoplasia of the cochlear branch.
The otoacoustic emission is a sound created by the healthy cochlea and measured in the external auditory canal. It occurs spontaneously (spontaneous otoacoustic emission) and during or after external acoustic stimulation (evoked otoacoustic emission). The outer hair cells in the cochlea, considered to be responsible for the sharp tuning and exquisite sensitivity of the ear, are thought to be the dominant source of otoacoustic emissions in mammals from animal studies (6). The distortion product otoacoustic emission is one of the evoked otoacoustic emissions. Two simultaneously presented pure tones evoke the distortion-product otoacoustic emission response, the levels of which correlate with the degree of damage in the cochlea (7). Our patient had documented severe impairment of the left cochlea and the cochlear branch of the eighth cranial nerve.
One possible explanation for the MR findings is that mumps infection resulted in the atrophy of the cochlear branch. Viral infections, such as mumps, measles, chickenpox, rubella, and influenza, are well-recognized causes of postnatal hearing loss. Mumps is supposed to be one of the most common causes of acquired unilateral SNHL in children (8). It is postulated that viruses reach the inner ear via the circulatory route, via the meningeal route, or from the middle ear. Mumps deafness is usually sudden in onset, profound, and unilateral. It occurs in less than 0.1% of mumps cases. Because of the normal hearing in the unaffected side, deafness in children is sometimes not recognized until a survey is performed for hearing impairment. Pathologically, mumps affects the organ of Corti, stria vascularis, tectorial membrane, and Reissner membrane. Loss of nerve fibers and spiral ganglion cells is also seen (9). The MR findings and the results of the otologic examination in our case might be secondary to these pathologic changes. To our knowledge, no reports documenting atrophy of the cochlear nerve owing to viral infection have included imaging studies.
Another possible explanation for the reduced size of the cochlear branch is developmental malformation. In our case, the left IAC at the fundus seemed to be slightly narrower than that of the contralateral side. Casselman et al (5) proposed the classification of developmental anomalies. One of them was described as aplasia or hypoplasia of the cochlear branch of the VCN with the normal labyrinth. They also hypothesized that loss of a sufficient number of neuronal fibers of the VCN may result in stenosis of the IAC during embryologic development. The findings of our case may be explained by this hypothesis.
Epidemiologic studies suggest that approximately one third of all cases of childhood hearing impairment are hereditary, one third are acquired and have causes for the malfunction in the auditory system with reasonable assurance, and the remaining cases are idiopathic (10). High-resolution T2-weighted MR imaging may disclose abnormalities in some of the children with SNHL, corroborating or providing presumptive evidence of the cause of SNHL. Clinically, detection of the VCN abnormality is beneficial to the selection of candidates for cochlear implantation (5). In our case, it could be predicted that cochlear implantation in the left ear would result in poor hearing ability.
Conclusion
A reduced size of the cochlear branch of the VCN was delineated by 3DFT-CISS images in a patient with unilateral SNHL. We hypothesize that previous mumps infection or developmental malformation might explain this finding. High-resolution T2-weighted images may help determine the cause of SNHL in children. Clinically, detection of the VCN abnormality is beneficial to the selection of candidates for cochlear implantation.
Footnotes
Address reprint requests to Susumu Furuta, MD, Department of Diagnostic Radiology, Tohoku University School of Medicine, 1-1, Seiryo-machi, Aoba-ku, Sendai, 980-8574, Japan.
References
- 1.Casselman JW, Kuhweide R, Deimling M, Ampe W, Dehaene I, Meeus L. Constructive interference in steady state-3DFT MR imaging of the inner ear and cerebellopontine angle. AJNR Am J Neuroradiol 1993;14:47-53 [PMC free article] [PubMed] [Google Scholar]
- 2.Rubinstein D, Sandberg EJ, Gajede-Law AG. Anatomy of the facial and vestibular nerves in the internal auditory canal. AJNR Am J Neuroradiol 1996;17:1099-1105 [PMC free article] [PubMed] [Google Scholar]
- 3.Casselman JW, Kuhweide R. Ampe W, et al. Inner ear malformations in patients with sensorineural hearing loss: detection with gradient-echo (3DFT-CISS) MRI. Neuroradiology 1996;38:278-286 [DOI] [PubMed] [Google Scholar]
- 4.Kim H, Kim D, Chung I, Lee W, Kim K. Topographical relationship of the facial and vestibulocochlear nerves in the subarachnoid space and internal auditory canal. AJNR Am J Neuroradiol 1998;19:1155-1161 [PMC free article] [PubMed] [Google Scholar]
- 5.Casselman JW, Offeciers FE, Govaerts PJ, et al. Aplasia and hypoplasia of the vestibulocochlear nerve: diagnosis with MR imaging. Radiology 1997;202:773-781 [DOI] [PubMed] [Google Scholar]
- 6.Trautwein P, Hofstetter P, Wang J, Salvi R, Nostrant A. Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hear Res 1996;96:72-82 [DOI] [PubMed] [Google Scholar]
- 7.Probst R, Hauser R. Distortion product otoacoustic emissions in normal and hearing-impaired ears. Am J Otol 1990;11:236-243 [DOI] [PubMed] [Google Scholar]
- 8.Paparella MM, Schachern PA. Sensorineural hearing loss in children: nongenetic. In: Paparella MM, Shumrick DA, Gluckman JL, Meyerhoff WL, eds Otolaryngology 3rd ed. Philadelphia: W.B. Saunders Company; 1991:1579-1599 [Google Scholar]
- 9.Smith GA, Gussen R. Inner ear pathologic features following mumps infection. Arch Otolaryngol 1976;102:108-111 [DOI] [PubMed] [Google Scholar]
- 10.Grundfast KM. Hearing loss. In: Bluestone CD, Stool SE, Kenna MA,eds Pediatric Otolaryngology 3rd ed. Philadelphia: W.B. Saunders Company; 1996:249-311 [Google Scholar]


