Abstract
BACKGROUND AND PURPOSE: The traditional endovascular approach to a cerebral aneurysm is anterograde, with the embolization and balloon protection catheters introduced via the parent vessel. Unfortunately, this approach may be restrictive, because these catheters cannot always be navigated at an optimal angle into the arterial branch that needs balloon protection or the part of the aneurysm that needs coiling. The purpose of this study was to determine the efficacy of a retrograde approach.
METHODS: Twelve patients, seven women and five men, 28 to 65 years old (mean age, 45 years), were treated via the retrograde approach between March 1998 and February 1999. Three patients were treated for acutely ruptured aneurysms following subarachnoid hemorrhage. The rest had asymptomatic, unruptured aneurysms.
RESULTS: We were able to accomplish endovascular treatment in 10 cases. In the other two, the attempted retrograde route of access could not be achieved. The treatment afforded complete embolization in nine of the 10 patients. Symptomatic distal clot embolization occurred in one patient who had some residual, albeit improving, deficits at discharge. No other patients worsened with the treatment. There were two intraprocedural aneurysmal ruptures. None of the aneurysms restudied within 6 months (eight of 12) showed evidence of recanalization.
CONCLUSION: Our results indicate that it is possible to safely and effectively access a cerebral aneurysm via a retrograde approach. We believe that the anatomic benefits afforded by this technique outweigh the potential risks associated with the catheterization of another major cerebral arterial feeder.
Endosaccular embolization of cerebral aneurysms with the use of Guglielmi detachable coils (GDCs) provides both short- and intermediate-term protection from subarachnoid hemorrhage with low morbidity (1). Current catheter technology affords access to almost all cerebral aneurysms, provided that the proximal vessels are not too diseased or tortuous. Indeed, with balloon protection (2) or stenting (3–6), even wide-necked lesions can be successfully treated. Generally, the approach to aneurysms has been anterograde, with the microcatheter for coil introduction and the balloon catheter for neck protection or stenting entering via the parent vessel feeding the aneurysm. Sometimes this approach is not optimal, either for filling the aneurysmal sac or for “remodeling” the neck. Also, persisting symptoms from aneurysms treated by parent vessel occlusion may rule out an anterograde approach.
It is now possible to access cerebral aneurysms by using an approach other than the usual anterograde route through the parent artery; ie, via a communicating vessel (7). We describe the indications, technique, and short-term results in a small group of patients with cerebral aneurysms who were treated via this approach. It is our belief that this procedure, often used in conjunction with the balloon remodeling technique, permits coiling of some otherwise uncoilable aneurysms and leads to improved anatomic results.
Methods
Patient Population
Twelve patients, seven women and five men, 28 to 65 years old (mean age, 45 years), were treated for cerebral aneurysms via the retrograde approach between March 1998 and February 1999. Three patients were treated for acutely ruptured aneurysms following subarachnoid hemorrhage. The rest had asymptomatic, unruptured aneurysms.
Aneurysmal Characteristics
Aneurysms were located in both the posterior and anterior circulations, and all, in the opinion of the senior author, had configurations that required a retrograde approach, using either the embolization or balloon protection catheter for optimal management. The lesions were located on or upstream from the circle of Willis. Given the anatomic constraints dictated by percutaneous access to the cerebral circulation from the internal carotid artery (ICA) and vertebrobasilar systems, this technique was not considered for aneurysms of the middle cerebral artery (MCA) or those beyond the A1 and P1 segments of the anterior cerebral artery (ACA) and posterior cerebral artery (PCA), respectively. Open communicating arteries to the vessel harboring the aneurysm and a good proximal vessel, with minimal tortuosity, were required to successfully access the aneurysm in a retrograde fashion from a different circulation. Of note, two patients (cases 9 and 10) had undergone previous bilateral vertebral artery (VA) occlusions for difficult aneurysms in the area of the basilar bifurcation. These aneurysms had continued to grow and required further treatment. The retrograde approach was the only option for these patients.
Procedure
Anticoagulation
All patients received an intravenous heparin bolus of 5000 IU followed by a continuous infusion of 2500–3000 IU/h. The activated clotting time (ACT) was checked intermittently throughout the procedure and the heparin was titrated to keep the ACT close to 300 seconds. Those patients who had not suffered a recent subarachnoid hemorrhage also received an intravenous bolus of 250 mg of acetylsalicylic acid (ASA) at the start of the procedure. All patients were kept on subcutaneous fractionated heparin for 1 week and oral ASA for 1 month after the procedure.
Devices
We generally preferred the Tracker-10 microcatheter (Target Therapeutics/Boston Scientific, Fremont, CA) for coil delivery and the Solstice Balloon Occlusion System (Medtronic MIS, Sunnyvale, CA) for remodeling. While this system is no longer available commercially, several balloon catheters of similar design are under development by other manufacturers. Guidewires were carefully chosen according to the demands of the case. The Transend 0.014-inch wire (Target Therapeutics) for the Tracker-10 catheter was used in all cases, although we switched to a Terumo 0.012-inch guidewire (Terumo Corp, Tokyo, Japan) with a 90° angle when the navigation was unsuccessful with the Transend. For the Solstice balloon catheter, the standard Quicksilver wire, which is provided with the Solstice Balloon Occlusion System, was often adequate, although a Terumo 0.012-inch or Transend 0.010-inch wire was needed for more difficult navigations. We found that both these guidewires effectively closed the inner lumen of the Solstice catheter to allow for balloon inflation and deflation. As a general rule, we were flexible in terms of our choice of guidewire, not feeling constrained by manufacturers' recommendations. All aneurysms were embolized with GDC-10 microcoils (Target Therapeutics).
Technique
General anesthesia was induced in all cases. Patients then underwent full angiographic cerebral evaluations, which included a high-resolution 3D rotational study, using the Integris BN 5000 biplane system (Philips Medical Systems, Best, the Netherlands). Our general preference was for bifemoral catheterization with short introducer sheaths, followed by guiding catheters in the appropriate cervical trunks of the feeding arteries. All aneurysms in this series had a complex anatomy, with features such as wide necks, adjacent arterial branches, and irregularly shaped sacs. In all but three cases, both embolization and balloon protection catheters were required. A standard microcatheter technique, using biplane fluoroscopy with roadmapping, was employed. The remodeling technique has been described elsewhere (2).
Indications
As mentioned above, all aneurysms, in the senior author's opinion, had an anatomy that dictated an unconventional approach with a microcatheter. This was usually because an arterial branch adjacent to the aneurysm that needed balloon protection was at an angle that was best protected by a nonanterograde approach with the balloon protection catheter (Fig 1). Sometimes the technique was chosen for aneurysms that could not be completely embolized, because the catheter could not be navigated, via an anterograde route, into a critical part of the aneurysm (Fig 2).

fig. 1. Case 6.
A, Anteroposterior right vertebral angiogram shows a large wide-necked aneurysm at the basilar tip; B, lateral view of same; C, 3D reconstruction, lateral view, note width of neck in anteroposterior diameter; D, anteroposterior view of microcatheter, with coil ready for deployment, introduced into aneurysm via right VA; also visible is the remodeling balloon catheter with guidewire, introduced via the right ICA, then coursing through the PCom, right P1, and across the neck of the aneurysm, with the wire extending into the left P1 and P2; E, schematic illustration of catheter deployment; F, final phase of embolization, just before detachment of last coil (arrow on proximal marker of embolization catheter), with remodeling balloon inflated, anteroposterior view; G, lateral view of same, note how the top of the BA has been remodeled with the balloon, reconstructing the normal vascular lumen; H, final postembolization anteroposterior vertebral angiogram; I, same, lateral view, note the curve of the top of the BA and the proximal P1 due to balloon remodeling.
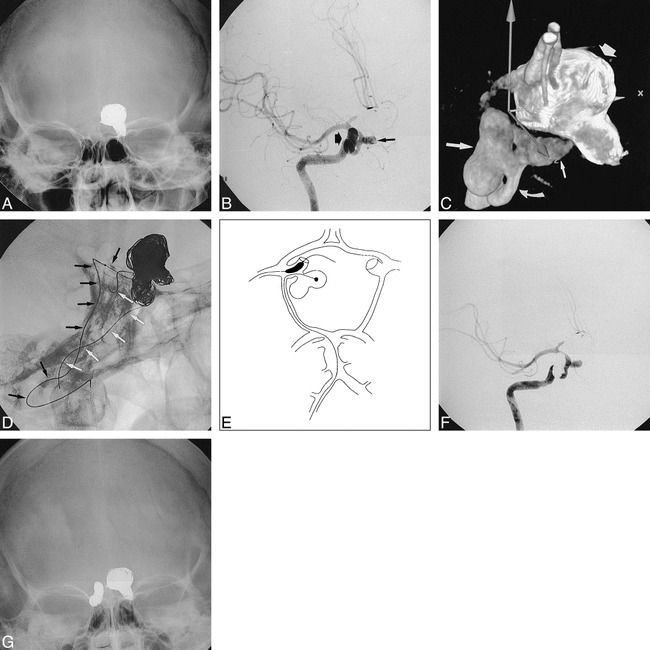
fig. 2. Case 4.
A, Skull radiograph, anteroposterior view, shows coils in previously embolized left ICA aneurysm; B, right anterior oblique projection of right ICA angiogram shows bilobate lateral-wall ICA aneurysm (wide arrow) and unilobar medial-wall aneurysm (thin arrow); C, 3D reconstruction, same view, shows bilobate lateral-wall aneurysm (large straight white arrow) and medial-wall aneurysm (small straight white arrow) of right ICA (curved arrow) as well as coil mass from embolized left ICA aneurysm (wide arrow), gray arrows are orientation markers from the reconstruction; D, same projection, higher magnification, shows nonsubtracted view of balloon and embolization catheters during embolization of anterior lobe of the lateral-wall aneurysm; the balloon catheter and wire are coming up the ICA and extending into the MCA (white arrows); the embolization catheter, which has a coil in it, comes up the right VA, into the BA, then into the right P1, PCom, ICA, and aneurysm (black arrows); E, schematic view shows course of balloon and embolization catheters in this case; F, post-embolization right ICA angiogram shows complete packing of the lateral wall ICA aneurysm; G, post-treatment skull radiograph, anteroposterior view, shows coils in right ICA bilobate aneurysm.
Clinical and Angiographic Follow-up
Each patient was scheduled for a follow-up angiogram at 3 months. A Glasgow Outcome Scale score was recorded for all patients at the time of discharge from the hospital (8).
Illustrative Cases
Case 6
A 50-year-old woman with a history of smoking was imaged because of headaches. An aneurysm at the basilar bifurcation was detected, and a subsequent angiogram showed that it had a wide neck (Fig 1). The patient was referred to our institution for endosaccular coiling. The bifurcation had a perfect “T” shape, with both P1 segments of the PCAs coming off the terminal basilar artery (BA) at right angles. Because of this orientation, and the fact that the patient had two good posterior communicating arteries (PCom), we believed we would achieve optimal positioning of the remodeling balloon by placing it across the tip of the BA, bridging one P1 to the other. Accordingly, after a guiding catheter had been placed in the right ICA, the balloon was navigated up the ICA into the right PCom, and then from the right P1 across the neck of the aneurysm into the left P1. The guidewire was positioned in the left P2 for stability. Then a catheter was inserted into the right VA and another microcatheter was introduced into the aneurysm. This orientation of the balloon allowed us to reposition the embolization catheter in front of and behind the balloon toward the end of the coiling, to effect an anatomic recreation of the normal anatomy. The patient suffered no ill effects from the procedure and was discharged from the hospital after 5 days.
Case 4
A 47-year-old woman had first presented 6 months earlier with a left-sided visual field defect. She was imaged and found to harbor a large supraclinoid ICA aneurysm on the left and two smaller ones on the right. The left-sided aneurysm was successfully coiled (Fig 2), and the patient returned for treatment of the right-sided lesion. It was thought that the larger, more irregular aneurysm on the lateral wall was the more dangerous one, so we next proceeded there. Because the aneurysm had a fairly large neck, a balloon catheter was introduced through a guiding catheter in the right ICA. Next, the aneurysm was inserted with a Tracker 10 catheter, via the same arterial route, and the aneurysm was coiled. The catheter naturally “fell” into the posterior and superior lobe of the aneurysm and good packing of this portion of the aneurysm was achieved. Only a few loops of a GDC went into the anterior and inferior lobe. Further efforts to reposition the microcatheter into this lobe were futile and it was believed that the coiling was not yet adequate. After further study of the anatomy, including a 3D angiogram, it was decided to approach the aneurysm from the posterior circulation. With the balloon catheter kept in the ICA, a guiding catheter was placed in the right VA and the embolization catheter was navigated up the BA, into the right P1, then to the right PCom, the right ICA, and directly into the anterior and inferior lobe of the aneurysm. The navigation was not facile, but upon arrival at the origin of the PCom, in the ICA, the catheter fell quite naturally into the anterior and inferior lobe of the aneurysm (Fig 2D). A rather dense packing with a good anatomic result was thereby achieved. The patient tolerated the procedure without complication. She was readmitted 2 months later, at which time the coils appeared stable, and the third aneurysm was embolized.
Results
Treatment Efficacy
No meaningful statistical analysis is possible with such a small number of patients. The data are presented in the Table.
Data in 12 patients with cerebral aneurysms treated via nonanterograde approach

In two patients, the retrograde catheterization failed. In one of these patients (case 11), the aneurysm was reaccessed, at the same sitting, from a conventional anterograde approach and was successfully embolized. In the other patient (case 9), the procedure was aborted because a small, asymptomatic, intraprocedural rupture occurred. The patient will be readmitted in the near future for another attempt.
Of the 10 aneurysms that were successfullyaccessed via this approach, 80% were completely occluded at the time of treatment. One patient in whom embolization was incomplete (case 5) had a particularly challenging aneurysm, the treatment of which had to be aborted before total occlusion could be achieved. This was because the aneurysm ruptured intraoperatively and the patient then suffered distal clot embolization. The other incompletely treated patient (case 10) had a difficult anatomy, owing to a dissecting aneurysm of the left superior cerebellar artery. Because he had undergone previous bilateral VA occlusions, it was considered too dangerous to insert catheters into both PComs, one for the embolization catheter the other for a remodeling balloon. Since the patient was dependent on his upper BA for perfusion of the posterior fossa, and we could not protect it with a balloon, it was decided that an aggressive coiling in the area of the aneurysm was best avoided. Of interest, the aneurysm eventually thrombosed completely (see below).
Although no data are available on the stability of the embolizations in this series, there is no reason to believe that it should be different from that seen after more conventional, anterograde remodeling (2).
Complications Related to Technique
Thromboembolic Events
One patient (case 5) suffered a thromboembolic event as the result of treatment. This occurred in the territory of the vessel upon which the aneurysm was located, after a small intraoperative aneurysmal rupture required reversal of heparin. There were no embolic events in the territory of the vessel used for access for the nonanterograde route of access.
Rupture of Aneurysmal Sac
Two intraoperative ruptures occurred (cases 5 and 9). The hemorrhages were small and easily controlled in both cases. One patient (case 5) deteriorated as a result of a distal thromboembolism after his heparin was reversed. The aneurysm in this case was already partially coiled and the bleeding was stopped with the aid of the remodeling balloon. The other patient suffered no ill effect. The small perforation in the aneurysm in this case was caused by a 0.010-inch guidewire during initial catheterization of the aneurysm. The small rupture sealed rapidly, although the heparin had to be antagonized and the procedure aborted.
Clinical Outcome
Only one patient (case 5) deteriorated as a result of treatment. He awoke with dysphasia and right-sided hemiparesis that gradually improved over the following days. While he left the hospital under his own locomotion 2 weeks after treatment, he had some residual right-sided hemiparesis and dysphasia. No aneurysmal ruptures occurred in any of the treated patients.
Follow-up Angiography
At the time of this writing, eight of the 12 patients have undergone early follow-up angiography. All lesions that were 100% occluded at the end of treatment remained so (six of six). In one patient (case 10), a complex dissecting aneurysm in the region of the left superior cerebellar artery, previously treated with bilateral VA occlusion, was considered to be only 80% occluded at the end of treatment. Follow-up angiography, however, showed that the aneurysm had become completely thrombosed. The other patient in whom the original coiling procedure had to be aborted before any coils could be deployed (case 9) expectedly showed a persistent aneurysm when she was restudied several months later.
Discussion
Treatment Feasibility
This series confirms that it is possible to access an aneurysm using an unconventional approach, crossing over from another major cerebral artery via a communicating artery. This allows for optimal GDC coiling and, if necessary, balloon remodeling.
Treatment Efficacy
The technique described was developed to allow for optimal treatment of aneurysms that otherwise were difficult or impossible to access. Given the success rate of embolization in the population studied (nine total occlusions of 12 aneurysms), it seems that the treatment is efficacious. In some patients, a retrograde approach afforded embolization that was otherwise impossible, while in others it allowed for a superior, more complete packing. Given experimental evidence that partial aneurysmal packing is not as protective against rupture as complete packing (9), it seems justifiable to pursue techniques that enable a denser endosaccular embolization to be achieved. It is difficult, however, to improve upon the complication rate from surgery for nonruptured aneurysms in good hands (10, 11). This new approach to embolization should probably, therefore, be reserved for operators with considerable experience in the endovascular treatment of aneurysms.
Complications Related to the Technique
Thromboembolic Events
The reason for the clots in our one patient is certainly the reversal of heparinization, which was necessitated by a small intraoperative aneurysmal rupture.
Rupture of the Aneurysmal Sac
Our experience indicates that there is minimal direct morbidity when an aneurysm ruptures during coiling if a remodeling balloon is in place (2). As the bleeding was easily controlled in both our cases of intraoperative rupture, and the total amount of subarachnoid clot was small, we do not think that the subarachnoid blood was the direct cause of clinical deterioration in either case. The chain of events that led to a less than good outcome in case 5, however, was set in motion by the rupture, which necessitated the reversal of heparin, which subsequently led to the development of thromboembolism and a stroke. The patient had a partially coiled, wide-necked aneurysm that was undoubtedly thrombogenic. Most cases of intraprocedural rupture with a protection balloon in place, however, have had good outcomes in our experience. Although the 17% rate of aneurysmal rupture in this series (two of 12) may raise concerns, the numbers are too small to warrant any conclusions. Furthermore, we do not think that the risk of aneurysmal rupture is significantly elevated by this procedure. Indeed, the risk of a devastating, uncontrollable hemorrhage is probably lessened, as this technique allows us to optimally position the remodeling balloon so that it can be inflated in an emergency to control the bleeding.
Indications for Use of the Technique
Considerable experience with GDC embolization of aneurysms is necessary to be able to judge when a nonanterograde approach is indicated. We have found that 3D angiography provides valuable anatomic information that helps us to decide at the outset if an aneurysm is able to be coiled and, if it is, to choose an appropriate approach. High-resolution digital subtraction angiography is also necessary to assess the circulation to determine if a nonanterograde approach is even possible. The communicating arteries do not have to be huge, but they should be angiographically determined to be open, and larger than the device to be navigated, before an attempt at navigation is made. A nonanterograde approach should only be considered for those cases in which the anterograde route will not provide adequate access for coiling and remodeling. If the operator does not feel confident or experienced enough to attempt an approach from another vessel, then the patient is perhaps best referred to a surgeon for an opinion on the feasibility of an open approach.
Conclusion
While this technique demands that a catheter be inserted into second major cervical arterial feeder to the brain, with a potential for ischemic complications in another part of the brain, significant advantages in terms of access and anatomic reconstruction are achieved, allowing some otherwise impossible aneurysms to be embolized. With our protocol of heparinization, and a careful microcatheter technique, we do not think that such catheterizations are particularly dangerous. Perhaps newer embolization products, such as cellulose acetate and ethylene vinyl alcohol polymers (12–14), may eventually be easier to deliver than coils, and not demand unconventional routes for their application. For the time being, however, as this technique increases the options for endovascular treatment of cerebral aneurysms, its use should be considered in selected cases. As navigators, and indeed explorers, of the cerebral vasculature, neurointerventionalists should remember the old adage that how one gets to a destination is just as important as, and sometimes more satisfying than, what one does upon arrival.
Footnotes
Support was provided to Ian Ross by the Department of Surgery, University of Manitoba, Winnipeg, Canada, and by the Royal College of Physicians and Surgeons of Canada.
Address reprint requests to Professor Jacques Moret, Service de Neuro-radiologie Interventionnelle, Fondation Ophtalmologique Rothschild, 25–29 rue Manin, 75940 Paris, Cedex 19, France.
References
- 1.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475-482 [DOI] [PubMed] [Google Scholar]
- 2.Moret J, Cognard C, Weill A, Castings L, Rey A. The “remodeling technique” in the treatment of wide neck intracranial aneurysms: angiographic results and clinical follow-up in 56 cases. Interv Neuroradiol 1997;3:21-35 [DOI] [PubMed] [Google Scholar]
- 3.Higashida RT, Smith W, Gress D, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. J Neurosurg 1997;87:944-949 [DOI] [PubMed] [Google Scholar]
- 4.Lylyk P, Ceratto R, Hurvitz D, Basso A. Treatment of a vertebral dissecting aneurysm with stents and coils: technical case report. Neurosurgery 1998;43:385-388 [DOI] [PubMed] [Google Scholar]
- 5.Mericle RA, Lanzino G, Wakhloo AK, Guterman LR, Hopkins LN. Stenting and secondary coiling of intracranial internal carotid artery aneurysm: technical case report. Neurosurgery 1998;43:1229-1234 [DOI] [PubMed] [Google Scholar]
- 6.Sekhon LHS, Morgan MK, Sorby W, Grinnell V. Combined endovascular stent implantation and endosaccular coil placement for the treatment of a wide-necked vertebral artery aneurysm: technical case report. Neurosurgery 1998;43:380-384 [DOI] [PubMed] [Google Scholar]
- 7.Gurian JH, Viñuela F, Gobin YP, Watson VE, Duckwiler GR, Guglielmi G. Aneurysm rupture after parent vessel sacrifice: treatment with Guglielmi detachable coil embolization via retrograde catheterization: case report. Neurosurgery 1995;37:1216-1221 [DOI] [PubMed] [Google Scholar]
- 8.Jennett B, Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet 1975;1:480-484 [DOI] [PubMed] [Google Scholar]
- 9.Byrne JV, Hubbard N, Morris JH. Endovascular coil occlusion of experimental aneurysms: partial treatment does not prevent subsequent rupture. Neurol Res 1994;16:425-427 [DOI] [PubMed] [Google Scholar]
- 10.King JT, Berlin JA, Flamm ES. Morbidity and mortality from elective surgery for asymptomatic, unruptured, intracranial aneurysms: a meta-analysis. J Neurosurg 1994;81:837-842 [DOI] [PubMed] [Google Scholar]
- 11.Solomon RA, Fink ME, Pile-Spellman J. Surgical management of unruptured intracranial aneurysms. J Neurosurg 1994;80:440-446 [DOI] [PubMed] [Google Scholar]
- 12.Kinugasa K, Kimata I, Hirotisune N, et al. Early treatment of subarachnoid hemorrhage after preventing rerupture of an aneurysm. J Neurosurg 1995;83:34-41 [DOI] [PubMed] [Google Scholar]
- 13.Nishi S, Taki W, Nakahara I, et al. Embolization of cerebral aneurysms with a liquid embolus, EVAL mixture: report of three cases. Acta Neurochir (Wien) 1996;138:294-300 [DOI] [PubMed] [Google Scholar]
- 14.Terada T, Nakamura Y, Nakai K, et al. Embolization of arteriovenous malformations with peripheral aneurysms using ethylene vinyl alcohol copolymer: report of three cases. J Neurosurg 1991;75:655-660 [DOI] [PubMed] [Google Scholar]


