Abstract
BACKGROUND AND PURPOSE: MR studies have shown hippocampal atrophy to be a sensitive diagnostic feature of Alzheimer's disease (AD). In this study, we measured the hippocampal volumes of patients with a clinical diagnosis of normal pressure hydrocephalus (NPH), a potentially reversible cause of dementia when shunted. Further, we examined the relationship between the hippocampal volumes and cortical AD pathologic findings, intracranial pressure, and clinical outcomes in cases of NPH.
METHODS: We measured hippocampal volumes from 37 patients with a clinical diagnosis of NPH (27 control volunteers and 24 patients with AD). The patients with NPH underwent biopsy, and their clinical outcomes were followed for a year.
RESULTS: Compared with those for control volunteers, the findings for patients with NPH included a minor left-side decrease in the hippocampal volumes (P < .05). Compared with those for patients with AD, the findings for patients with NPH included significantly larger hippocampi on both sides. Although not statistically significant, trends toward larger volumes were observed in patients with NPH who had elevated intracranial pressure, who benefited from shunting, and who did not display cortical AD pathologic findings.
CONCLUSIONS: Measurements of hippocampal volumes among patients with a clinical diagnosis of NPH have clear clinical implications, providing diagnostic discrimination from AD and possibly prediction of clinical outcome after shunting.
Normal pressure hydrocephalus (NPH) is a syndrome, the key features of which include the triad of gait disturbances, urinary incontinence, and memory loss in combination to varying extents (1, 2). The memory loss in cases of NPH is often slight or of moderate degree but may also be progressive and may dominate the clinical picture. Shunting may reverse the course of NPH, which distinguishes this syndrome from other types of primary degenerative dementia. Nevertheless, the overall outcome of shunting has left much to be desired. This has raised suspicions that concomitant pathologic abnormalities of the primary degenerative dementias, such as Alzheimer's disease (AD), which are not uncommon in the elderly, might modulate or complicate the clinical picture and outcome of NPH (3).
The hippocampus is a part of the medial temporal lobe memory system, responsible particularly for modulation, consolidation, and storage of newly acquired data. The hippocampus also exhibits characteristic pathologic features in AD even during the very earliest stages of the disease, whereas cognitively normal aging may have little, if any, effect on hippocampal volumes, neuronal histoarchitecture, or neuropsychologic domains considered to be related with the hippocampal function (4). Previous volumetric MR imaging studies have also shown that hippocampal atrophy can reliably show the distinction between dementia due to AD from cognitively normal aging (4, 5) and benign memory impairment unrelated to dementia (4). Nevertheless, this atrophy may not be specific to dementia in AD but may also take place in other dementias (6, 7). Therefore, important dimension to obtain when working to establish the diagnostic value of these measurements is hippocampal volume in other conditions that can cause dementia, including NPH.
In the present study, we measured the hippocampal volumes from MR images of patients with a clinical diagnosis of NPH. These were compared with the volumes of both healthy control subjects and AD patients. The aims of the study were twofold: first, to evaluate possible hippocampal atrophy in cases of NPH, and second, to determine correlates of the hippocampal structural integrity in relation to cortical AD pathologic findings, increased intracranial pressure (ICP), and clinical outcomes after shunting.
Methods
Patients
A total of 88 participants were examined in the study: 37 patients with a clinical diagnosis of NPH, 24 patients fulfilling the NINCDS-ADRDA criteria of probable AD (8), and 27 cognitively normal control volunteers matched for age and gender. The patients with AD who were chosen for the study had been recently diagnosed, and these cases represented mild-to-moderate AD. The clinical characteristics of the study groups are displayed in Table 1. The local research ethics committee approved the study. All participants provided informed consent for participation in the study after receiving an explanation of the study protocol.
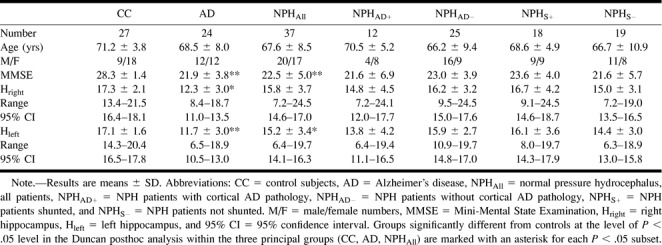
TABLE 1:
Clinical characteristics and normalized hippocampal volumes in the study groups, including the NPH subgroups
During a 2-year period from November 1, 1993, to December 31, 1995, all patients younger than 75 years who were referred to the university hospital having suspected NPH as a working diagnosis underwent general physical and clinical neurologic examinations and were prospectively evaluated with the study protocol. The diagnosis was based on the triad of symptoms found during the clinical neurologic examination, supplemented with CT findings (ventriculomegaly suggesting increased ICP without any specific pathologic abnormalities blocking the CSF flow). Apraxia of gait had to be present in the clinical neurologic examination. The history of urinary incontinence was gained from an interview with the patient or a knowledgeable informant. The exclusion criteria for the study were malignant disease of any kind, diabetes mellitus for more than 5 years, and any severe vascular disease (systemic or cerebrovascular). Those participants who fulfilled the criteria underwent neuropsychologic testing, 24-hour ICP measurement, infusion testing, and MR imaging. The neuropsychologic testing included a battery of tests assessing learning abilities and memory, verbal and visuoconstructional abilities, and attention and flexibility of mental processing.
A cortical biopsy for neuropathologic evaluation was obtained from the right frontal lobe before inserting the ICP catheter for all participants with a clinical diagnosis of NPH. Cylindrical biopsy specimens with a diameter of 2 to 5 mm were fixed in formalin and embedded in paraffin, and 5-μm-thick sections were prepared for analysis. Hematoxylin and eosin staining was used for general evaluation of the preparations, and Bielschowsky silver and Congo stainings were used for evaluation of Alzheimer's degenerative changes. The classification of biopsies as being AD-positive or -negative was based on the presence of plaques on silver staining. For further accuracy, τ-protein and β-amyloid load were evaluated by standard immunohistochemical methods using a monoclonal antibody to τ-protein (AT-8, BR-003; Innogenics, Temse, Belgium), in a dilution of 1:500, or to β-amyloid (M872; Dako, Glostrup, Denmark), in a dilution of 1:100. The expression of the τ-protein was rated as negative or positive. The amount of staining for β-amyloid was evaluated semiquantitatively with the Quantimet 570 Image Analysis System (Leica Cambridge Ltd., Cambridge, England). In the image analysis, leptomeninges and crushed material were excluded, and the extent of staining was evaluated at ×100 magnification as counts of lesions, stained area, and stained area fraction.
For the ICP measurement, the basal level was set as the level of the forehead. A basal pressure below 5 mm Hg was considered normal. The ICP was considered abnormally high if it was above 10 mm Hg or if the basal pressure was between 5 and 10 mm Hg and there were any A-waves or more than 30% B-waves during the recording period. ICP of 20 mm Hg or more excluded the diagnosis of NPH. A shunt operation was performed in 18 of 37 participants fulfilling these criteria of elevated ICP, two of whom fulfilled the criteria for elevated basal pressure and 16 of whom met the criteria for pathologic pulse waves. All were shunted using similar ventriculoperitoneal Holter valve shunts.
The patients with NPH who underwent shunting were examined at two follow-up times: 3 months and 1 year postoperatively. Clinical and neuropsychological evaluations were performed to assess clinical outcome and efficacy of shunting. Palpation and CT confirmed shunt functioning. At the final follow-up, gait disturbances, incontinence, memory impairment, and activities of daily living (functional capacity, dependence on assistance) were subjectively classified as improved, unaltered, or deteriorated in consensus with the clinician, a relative, and, whenever possible, the patient. This rating was done without any knowledge of the MR or biopsy findings.
Control Subjects
The control volunteers were derived from our previous studies. Briefly, all underwent general physical and clinical neurologic examinations, comprehensive neuropsychological testing, an extensive battery of laboratory tests, EEG and event-related potentials, CT, single photon emission CT, and MR imaging of the brain. They were healthy and had no history of neurologic, psychiatric, or major systemic diseases. The investigation has been described elsewhere in detail (4).
MR Imaging
The participants underwent imaging with a 1.5-T Magnetom (Siemens, Erlangen, Germany) using a standard head coil and a tilted coronal 3D gradient-echo sequence (magnetization prepared rapid acquisition gradient-echo) with 10/4 (TR/TE) and one acquisition. The boundaries of the hippocampus were manually traced from coronal 2.0-mm-thick slices, oriented perpendicular to the long axis of the hippocampus. Tracing was performed within a short interval by a single rater who was blinded to the clinical and pathologic data. The rostral end of the hippocampus, when it first appeared below the amygdala, was the starting point. The caudal end of the hippocampus was taken as the section in which the crura of the fornices departed from the lateral wall of the lateral ventricles. The number of voxels within the region creating the volume was calculated by using custom made software on a standard Siemens work console. Hippocampal measurements were not used as a selection criterion for any of the study groups. To exclude confounding effects of individual and gender-related size variability, the volumes were normalized to a coronal intracranial area measured at the level of the anterior commissure. The methodology and reproducibility of the technique have been described in detail elsewhere (4).
Statistical Analyses
Volumes normalized for the intracranial area were used in all statistical analyses. One-way analysis of variance was used to compare the means over the study groups. The Duncan post hoc analysis was used to evaluate the statistical difference between the groups. The results are expressed as mean ± SD. The level of statistical significance of difference was P < .05.
Results
The clinical characteristics of the study groups are presented in Table 1. There were no differences in age or gender between the principal study groups. As expected, Mini-Mental State Examination (MMSE) scores were significantly higher in control volunteers than in patients (F[2.85] = 22.2, P < .0001). There were no significant differences between the NPH and AD groups regarding MMSE scores.
The normalized hippocampal volumes (Table 1) differed significantly in the three principal study groups on both sides (right, F = 17.5, left F = 23.5; P < .0001). In the post hoc analysis, the hippocampus on the right was significantly smaller in the AD group compared with the control and NPH groups (P < .05). No significant difference between the control volunteers and NPH patients emerged. Figure 1 displays a box plot showing median values, quartiles, and extreme values of the right hippocampal volumes within the principal study groups. The result was similar on the left side, except in the post hoc analysis, in which both the NPH (P < .05) and the AD (P < .001) groups were found to have smaller hippocampal volumes compared with the control group.
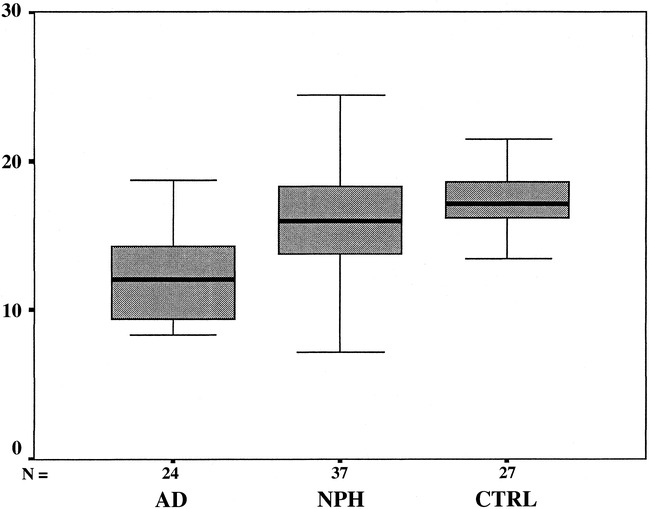
Fig 1. Box plot displaying median values, quartiles, and extreme values of the right normalized hippocampal volumes within the principal study groups. AD, measurements from patients with Alzheimer's disease; NPH, measurements from patients with normal pressure hydrocephalus; CTRL, measurements from control volunteers
The NPH group was then divided for further analysis into two subgroups according to neuropathologic findings based on biopsy and ICP measurements. The hippocampal volumes in these subgroups are also presented in Table 1.
Of the 37 participants who underwent biopsy, 25 (67.6%) were classified as being AD-negative, having no AD changes with silver stains. Two of these participants, however, displayed immunoreactivity for β-amyloid and from one participant, there was not sufficient material with which to perform immunohistochemistry. Twelve participants (32.4%) displayed cortical AD pathologic findings on silver staining. All 12 displayed immunoreactivity for β-amyloid, and two of them also displayed immunoreactivity for τ-protein. No significant difference was found when the hippocampal volumes were compared between the group with and the group without cortical AD pathologic findings. Similarly, no significant differences were detected in age, gender, or MMSE scores within these groups (Table 1).
In all, 18 (48.6%) patients had elevated ICP and underwent shunting. Of the patients who underwent shunting, only three (16.7%) displayed cortical AD pathologic findings, whereas among the 19 patients who did not undergo shunting, AD pathologic findings were found in nine (47.4%). When the patients with NPH were grouped according to whether they had undergone shunting, no significant statistical differences were found in age, gender, MMSE scores, or hippocampal volumes (Table 1).
The outcome of shunting in terms of improvement, no change, or deterioration of gait disturbance, incontinence, memory impairment, and activities of daily living and their relationship to hippocampal volumes and cortical AD pathologic findings are shown in Table 2. The only statistically significant finding related to the hippocampal volume was that for memory impairment. Only in that group, in which the memory status remained unaltered, were the hippocampal volumes significantly larger (F[2.15] = 5.1, P < .05) than those in the two other groups.
TABLE 2:
Normalized hippocampal volumes ± SD and cortical Alzheimer's disease in the 18 shunted patients in relation to clinical outcome 1 year after shunting
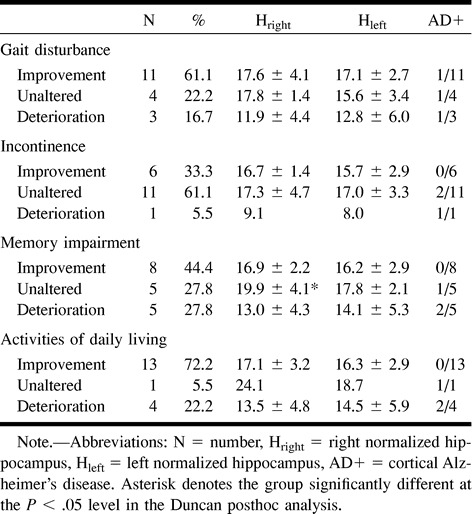
From Table 2, it may also be observed that the three patients who had undergone shunting and who had cortical AD pathologic findings had either unchanged or deteriorated outcomes, except for gait disturbance, which was improved in one patient with AD pathologic findings.
Discussion
In this study, we measured the hippocampal volumes in cases of NPH and compared them with those of the two control groups representing both normal aging and AD. Further, the relationships between the hippocampal volumes and the findings in cortical biopsy, ICP measurements, and clinical outcomes in the patients with NPH were evaluated. It was found that the NPH group displayed slightly smaller hippocampal volumes than did the normal control group, but this finding was significant only on the left side. On the other hand, the AD group had significantly smaller hippocampal volumes compared with both the control and NPH groups on both sides. This finding may have clear clinical implications: in cases of suspected NPH versus AD, when facing the question of whether to shunt, measurement of the hippocampus may be of diagnostic help. It is relevant to note that patterns of ventriculomegaly may not differ between patients with NPH and those with AD (9).
When the NPH group was divided into subgroups according to biopsy findings, no statistically significant differences were found in the hippocampal volumes between those with signs of cortical AD pathologic findings and those without. If these participants truly were patients with AD, this finding would be difficult to explain. Nevertheless, in this study, changes in AD pathologic findings in a biopsy were merely suggestive of and not definite for AD. False-negative cases of AD also constitute a possibility, because the focus of AD is more temporal than frontal. This possibility seems unlikely, because in cases with clear ventriculomegaly, the manifestations of AD would likely have reached the frontal lobes as well.
Again, when the patients with NPH were subdivided according to increased ICP, and thus according to their need for shunting, no differences in the hippocampal volumes between the subgroups were found. When the hippocampal volumes were compared in the shunted subgroup according to outcomes, only one statistically significant difference was found. Individuals with unchanged memory impairment during the 1-year follow-up period had the largest hippocampal volumes. Herein, with respect to the clinical outcome, lies a possible weakness of the study, which is a largely subjective evaluation of the outcomes. On the other hand, the validity of these findings is supported in that they were obtained in consensus by the family affiliate and the clinician blinded to the MR and biopsy results.
Despite the lack of final statistical power, some trends seem to emerge if we observe the actual volumes within the subgroups. The hippocampal volumes were larger in the participants who were primarily diagnosed as having NPH, who had elevated ICP, who benefited from shunting, and who did not have cortical AD pathologic findings and may thus have reflected “true” cases of NPH. The hippocampal volumes were larger in those for whom shunting improved gait disturbance or incontinence, which most likely reflects preserved general brain integrity. Only in that group in which the memory status remained unaltered were the hippocampal volumes significantly larger than those in the two other groups. Although this finding seems unexpected, it is important to note that no deterioration occurred. It is also possible that the baseline memory status was relatively good. Conversely, there was a trend toward smaller hippocampal volumes in participants who had AD pathologic findings, whose conditions deteriorated during the follow-up period, and who did not have elevated ICP. This group may represent false-positive cases of NPH or cases with concomitant AD.
Nonetheless, there seemed to be a minor decrease in the hippocampal volumes even among patients who seemed to represent true cases of NPH. In a previous MR study that compared the hippocampal body size between control volunteers and patients with NPH, the hippocampus was found to be smaller on both sides (10). Issues related to methodologic and subject selection most likely explain this minor discrepancy. In that study, only a portion of the hippocampus was measured and no biopsy data to control for AD pathologic findings were used. In general, the cortical gray in MR images has previously been reported to remain largely unaffected by NPH (9). Yet, in an experimental study by Del Bigio and Bruni (11), ventriculomegaly was shown to alter the metabolism of the monoamine neurotransmitters in the hippocampus and later, with prolonged exposure to increased ICP, to result in signs of ischemic injury. These findings are to some extent supported by the single photon emission CT study conducted by Larsson et al (12) in which decreased blood flow in the hippocampus was observed among patients with NPH, with a subsequent increase in blood flow after shunting. Previously, the same group also reported worse clinical outcomes with shunting in relation to symptom duration (13). Therefore, it seems that the course of NPH may lead to hippocampal damage, but this course, at least in its earliest stages, is less severe than in the primary degenerative dementias and might be reversible when shunting is promptly performed. This is when observing intact hippocampus might be of great clinical aid.
In general, this study illustrates not only the heterogeneity of this syndrome (3), even when controlling for histopathologic diagnosis, but also the comparative rarity and tendency to overdiagnose NPH (14), at least in a prospective study setting. In this study, samples were not available to control for the possible confounding contribution of apolipoprotein E genotype on hippocampal volume (15) and its impact on recovery of the brain after an insult (16). Regarding the diagnosis, this heterogeneity is not surprising, considering that none of the symptoms, memory impairment, gait disturbances (17, 18), incontinence (19), or ventricular dilation (10, 20), are specific to NPH in comparison with either cognitively normal aging or dementia. In contrast to this, it is our opinion that the hippocampus is substantially resistant to the effects of cognitive normal aging (4, 21, 22) and, conversely, a highly sensitive indicator of primary degenerative dementia (4−7). Therefore, in cases of equivocal or suspected secondary dementias, measurement of the hippocampal volumes may offer a proxy to detect the presence of concomitant primary degenerative dementia.
In summary, the hippocampi are substantially spared in NPH, at least compared with AD, although minor atrophy of the hippocampus can be found among patients with a clinical diagnosis of NPH. This atrophy seems to take place in varying degrees, but it does seem to be less marked in patients with true NPH (ie, in those who have elevated ICP, who benefit from shunting, and who do not have cortical AD pathologic findings). Therefore, hippocampal volume measurements have potentially great diagnostic value in the differential diagnosis between NPH and primary degenerative dementia.
Footnotes
This study was supported by the Finnish Neurosurgical Association, North-Savo Fund of the Finnish Cultural Foundation (S.S.), the Maire Taponen Foundation (S.S., M.P.L.), the Research Council for Health of the Academy of Finland, the Finnish Neurology Foundation, Instrumentarium Research Foundation, and the Farmos Research Foundation (M.P.L.), and by an EVO grant from the Kuopio University Hospital.
Presented in part as a poster at the Sixth International Conference on Alzheimer's Disease and Related Disorders, 1998, Amsterdam, The Netherlands.
Address reprint requests to Sakari Savolainen, MD, Department of Neurosurgery, Kuopio University Hospital, POB 1777, 70211 Kuopio, Finland.
References
- 1.Adams RD, Fisher CM, Hakim S, Ojeman RG, Sweet WH. Symptomatic occult hydrocephalus with normal cerebrospinal fluid pressure: a treatable syndrome. N Engl J Med 1965;273:117-126 [DOI] [PubMed] [Google Scholar]
- 2.Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure: observations on cerebrospinal fluid dynamics. J Neurol Sci 1965;2:307-327 [DOI] [PubMed] [Google Scholar]
- 3.Tedeschi E, Hasselbalch SG, Waldemar G, et al. Heterogenous cerebral glucose metabolism in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 1995;59:608-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laakso MP, Soininen H, Partanen K, et al. MRI of the hippocampus in Alzheimer's disease: sensitivity, specificity and analysis of the incorrectly classified subjects. Neurobiol Aging 1998;19:23-31 [DOI] [PubMed] [Google Scholar]
- 5.Jack CR Jr, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology 1997;49:786-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frisoni GB, Laakso MP, Beltramello A, et al. Hippocampal and entorhinal cortex atrophy in frontotemporal dementia and Alzheimer's disease. Neurology 1999;52:91-100 [DOI] [PubMed] [Google Scholar]
- 7.Laakso MP, Partanen K, Riekkinen P Jr, et al. Hippocampal volumes in Alzheimer's disease, Parkinson's disease with and without dementia, and in vascular dementia: an MRI study. Neurology 1996;46:678-681 [DOI] [PubMed] [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of NINCDS/ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984; 34:939-944 [DOI] [PubMed] [Google Scholar]
- 9.Matsumae M, Kikinis R, Mórocz I, et al. Intracranial compartment volumes in patients with enlarged ventricles assessed by magnetic resonance-based image processing. J Neurosurg 1996;84:972-981 [DOI] [PubMed] [Google Scholar]
- 10.Golomb J, de Leon MJ, George AE, et al. Hippocampal atrophy correlates with severe cognitive impairment in elderly patients with suspected normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 1994;57:590-593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Bigio MR, Bruni JE. Silicone oil-induced hydrocephalus in the rabbit. Childs Nerv Syst 1991;7:79-84 [DOI] [PubMed] [Google Scholar]
- 12.Larsson A, Bergh AC, Bilting M, et al. Regional cerebral blood flow in normal pressure hydrocephalus: diagnostic and prognostic aspects. Eur J Nucl Med 1994;21:118-123 [DOI] [PubMed] [Google Scholar]
- 13.Larsson A, Wikkelso C, Bilting M, Stephensen H. Clinical parameters in 74 consecutive patients shunt operated for normal pressure hydrocephalus. Acta Neurol Scand 1991;84:475-482 [DOI] [PubMed] [Google Scholar]
- 14.Vanneste J, Augustijn P, Dirven C, Tan WF, Goedhart ZD. Shunting normal-pressure hydrocephalus: do the benefits outweigh the risks? a multicenter study and literature review. Neurology 1992;42:54-59 [DOI] [PubMed] [Google Scholar]
- 15.Lehtovirta M, Soininen H, Laakso MP, et al. SPECT and MRI analysis in Alzheimer's disease: relation to apolipoprotein E ϵ4 allele. J Neurol Neurosurg Psychiatry 1996;60:644-649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teasdale GM, Nicoll JAR, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet 1997;350:1069-1071 [DOI] [PubMed] [Google Scholar]
- 17.Bloem BR, Haan J, Lagaay AM, van Beek W, Wintzen AR, Roos RA. Investigation of gait in elderly subjects over 88 years of age. J Geriatr Psychiatry Neurol 1992;5:78-84 [DOI] [PubMed] [Google Scholar]
- 18.O'Keeffe ST, Kazeem H, Philpott RM, Playfer JR, Gosney M, Lye M. Gait disturbance in Alzheimer's disease: a clinical study. Age Ageing 1996;25:313-316 [DOI] [PubMed] [Google Scholar]
- 19.Sugiyama T, Hashimoto K, Kiwamoto H, et al. Urinary incontinence in senile dementia of the Alzheimer type (SDAT). Int J Urol 1994;1:337-340 [DOI] [PubMed] [Google Scholar]
- 20.DeCarli C, Kaye JA, Horwitz B, Rapoport SI. Critical analysis of the use of computer-assisted transverse axial tomography to study human brain in aging and dementia of the Alzheimer type. Neurology 1990;40:872-883 [DOI] [PubMed] [Google Scholar]
- 21.Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging 1995;16:591-606 [DOI] [PubMed] [Google Scholar]
- 22.Bigler ED, Blatter DD, Anderson CV, et al. Hippocampal volume in normal aging and traumatic brain injury. AJNR Am J Neuroradiol 1997;18:11-23 [PMC free article] [PubMed] [Google Scholar]


