Abstract
Summary: We present the case of a female patient who was studied with dynamic contrast-enhanced CT perfusion before and after carotid revascularization. Before treatment, there was decreased perfusion in the ipsilateral insula, which was shown to be resolved on the scan obtained 1 day after treatment, indicating the technical success of the revascularization. In the ipsilateral basal ganglia, there was delayed contrast agent clearance from the tissue, which was attributed to vasodilation; after revascularization, there remained a subtle stenotic effect. The observed changes in the dynamic CT perfusion study suggest that this technique may be a useful tool in the evaluation of patients with asymmetrical cerebral blood flow.
Judging the clinical significance of extracranial and intracranial vascular stenosis can be problematic. Vascular stenosis may cause brain infarction, chiefly by generating emboli to the brain, but rarely can produce infarction from a low flow state. In the latter state, the brain is said to have “misery perfusion” in which cerebral arterioles are maximally dilated, autoregulation is exhausted, and cerebral blood flow (CBF) begins to decline below normal values. Brain oxygen extraction is increased to sustain brain metabolism, and cerebral blood volume (CBV) is increased (1−3). Because brain tissue sustaining misery perfusion is at risk for infarction, rational decisions regarding the use of cerebral revascularization procedures could be based on quantitative or qualitative blood flow studies of the brain. Although the quantitative measurements of CBF provided by positron emission tomography and xenon-enhanced CT can be considered a standard of reference, the availability of these techniques is limited.
MR techniques used to evaluate cerebral perfusion of patients with carotid artery and middle cerebral artery (MCA) disease have been studied intensely. The value of parameters such as CBF, CBV, peak enhancement, time to peak enhancement, and mean transit time of a contrast agent through the brain tissue is well established (4−6). Recently, perfusion CT has been shown to be a reliable alternative to MR perfusion imaging for the early detection of stroke (7−10). Using unen-hanced and contrast-enhanced helical scans and a dynamic single-section acquisition during the administration of the contrast agent, Koenig et al (7) could calculate CBV and CBF and evaluate the dynamics of the contrast enhancement curve.
To highlight the usefulness of dynamic CT perfusion, we report a case of a female patient who was studied before and after carotid revascularization. The questions we wanted to answer were—can dynamic CT perfusion show perfusion deficits in a patient with critical carotid stenosis, and can it show any immediate treatment effect after carotid revascularization?
Patient History
The patient was a 78-year-old right-handed woman with hypertension and previous small-vessel pontine stroke who presented with an acute right MCA distribution stroke. Her left hemiparesis, rightward gaze deviation, and left hemiparesis resolved during the first day while she was being treated with IV-administered heparin. Duplex sonographic imaging revealed critical (>80%) right carotid artery stenosis at the internal carotid origin, which was confirmed by digital subtraction angiography, which revealed a critical stenosis (>90%) of the right carotid artery. The patient underwent placement of a carotid stent (SMART 8- × 40-mm stent, Cordis Endovascular), resulting in restoration of good internal carotid caliber and recanalization of the right external carotid artery. Heparin was discontinued, and the patient was treated with clopidogrel (75 mg per day) and aspirin (325 mg per day). After treatment, the patient's gaze deviation and hemiparesis returned but resolved 2 days after the procedure. At 6 weeks, the patient had normal results of a neurologic examination, and duplex sonographic imaging of the artery revealed a patent stent.
CT Protocol
The following CT protocol was performed before and 1 day after the carotid stenting procedure. The CT protocol consisted of five series, with data acquisition spanning approximately 15 minutes. First, a scout scan was performed. Second, an unenhanced helical study of the whole brain was obtained (table speed, 3 mm/s; pitch, 1; field of view, 25 cm; tube voltage, 120 kV; tube current, 240 mA; matrix, 512 × 512; standard algorithm). Third, a dynamic contrast-enhanced cine sequence (without table movement) was performed. The dynamic contrast-enhanced cine sequence was performed with section location at the level of the third ventricle. This was done to cover the MCA territory, including the insula and basal ganglia, as well as the vascular territory of the anterior cerebral artery and posterior cerebral artery. The technical parameters were section thickness, 5 mm; field of view, 25 cm; tube voltage, 120 kV; tube current, 100 mA; matrix, 512 × 512; standard algorithm, rotation time, 1 s; acquisition time, 30 s). This was performed with the administration of the contrast agent performed during the cine-sequence acquisition (volume, 50 mL; injection rate, 3 mL/s; IV needle, 18-gauge; delay to start of the cine-sequence, 9 s). Fourth, contrast-enhanced helical study of the whole brain was performed. Technical parameters and location were identical to those used in series 2. Contrast agent was administered at a volume of 100 mL at an injection rate of 3 mL/s, and the helical scan started with a delay of 20 s. Fifth, secondary reconstruction of series 4 with a soft algorithm and 2-mm intervals was obtained.
From the acquired data, subtraction images were created subtracting corresponding images of series 2 from series 4, yielding spatial maps of the change in Hounsfield units on a voxel-by-voxel basis. Dividing the density changes in tissue by the density changes in blood on a voxel-by-voxel basis was performed to normalize the tissue enhancement by the vascular enhancement and revealed fractional blood volume (fBV) maps covering the whole brain.
From series 3, irregularly shaped regions of interest were drawn in blood (anterior cerebral artery and sagittal sinus) as well as tissue areas of interest (insula, basal ganglia, whole MCA territory, anterior cerebral artery, and posterior cerebral artery territory) using the MRVision software package (The MRVision Company, Menlo Park, CA). These were investigated to reveal the contrast agent transit curve. Furthermore, from the obtained density values, the following parameters were calculated. The peak was the maximum density change (Hounsfield units) after the administration of contrast agent. The time to peak was the elapsed time from the start of the cine sequence to the peak CBV, which was the integral of the tissue Hounsfield unit curve divided by the integral of the blood Hounsfield unit curve. This postprocessing of the cine sequence (3) takes approximately 10 minutes on a UNIX workstation. From series 4, we created CT angiograms by using 3D surface rendering or maximum intensity projection algorithms or both.
Results from CT Perfusion Study
Before Revascularization
Unenhanced CT scans showed multiple lacunar infarcts in the right thalamus, left external capsule, and left lateral putamen. There was no mass effect, no edema, and, thus, no sign of a new infarction. The CT perfusion study revealed delayed and attenuated contrast enhancement in the right insula (Fig 1) caused by the carotid stenosis. This chronic ischemic effect was also reflected by a lower CBV on the right side compared with the left insula (right:left ratio, 83%; Table), as well as a higher and earlier peak (Table). In the right basal ganglia, the initial arrival of the contrast agent was not impaired compared with the contralateral side; however, the peak was greater on the right, and time to peak as well as washout were delayed (Table, Fig 2). This mechanism yields an increased CBV in the right basal ganglia compared with the contralateral side (right:left ratio, 119%; Table). The fBV maps revealed a slightly increased intensity in the whole right MCA territory (Fig 3), which was attributed to the vasodilation.
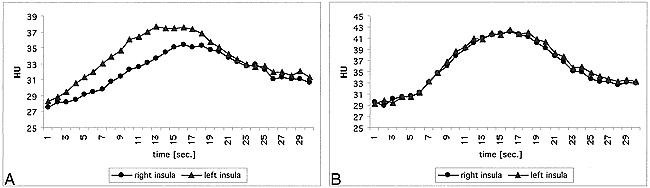
Fig 1. Enhancement in the right and left insula after injection of 50 mL iodinated contrast agent before and after carotid revascularization.
A, Before revascularization there is considerable delayed and attenuated enhancement in the right insula compared with the left.
B, After treatment, this differerence is resolved and the contrast agent transit curves are symmetrical.
Cerebral blood volumes (CBV), peak and time to peak for different anatomical areas before and after revascularization, calculated from the dynamic CINE CT acquisition
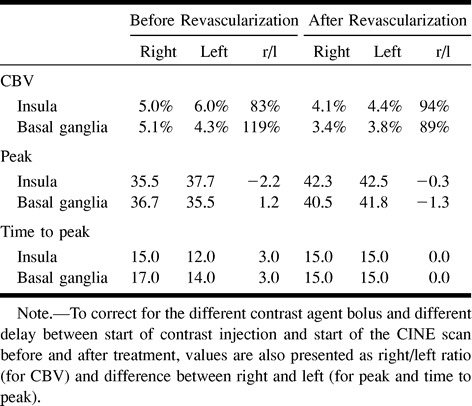
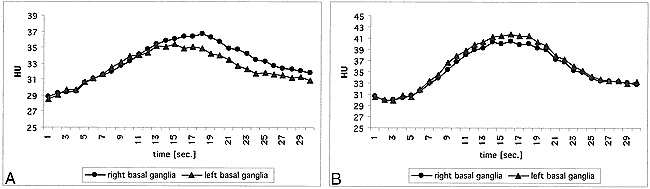
Fig 2. Enhancement in the right and left basal ganglia after injection of 50 mL iodinated contrast agent before and after carotid revasularization.
A, Before treatment, there is symmetric initial enhancement; however, the peak is later and higher and the washout is delayed on the right side compared with the left. This indicates loss in autoregulation and “pooling” of contrast agent.
B, The findings described in A are resolved 1 day after treatment, indicating reversal of vasodilation. A subtle stenotic effect on the right is indicated by a flatter curve and a lower contrast enhancement peak.
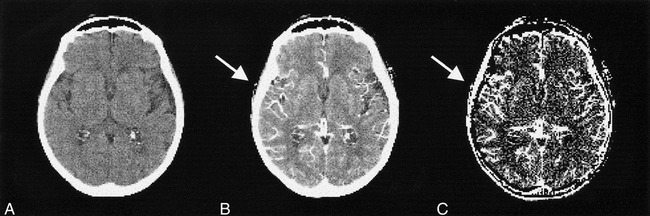
Fig 3. CT images before revascularization. Precontrast (A), postcontrast (B) (after 150 mL contrast agent administration), and calculated fBV maps (C) are shown. The vessels in the right MCA territory appear dilated (B, arrow), and there is an increased fBV in the same area (C, arrow)
After Revascularization
One day after dilation of the right carotid artery and stent placement, both the contrast transit curves (Fig 1) and the quantified parameters (Table) in the insula were similar on both sides. In the basal ganglia, there was a remaining subtle stenotic effect, reflected by the transit curve (Fig 2) and by the slightly lower CBV on the right side (right:left ratio, 89%) and the lower enhancement peak (Table). The spatial maps of the fBV showed a smaller area and lower intensity in the right MCA compared with the pretreatment maps (Fig 4).
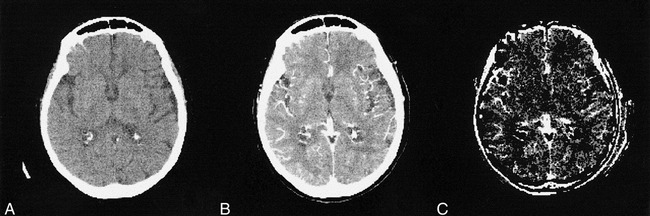
Fig 4. CT images after revascularization. Precontrast (A), postcontrast (B) (after 150 mL contrast agent administration), and calculated fBV maps (C) are shown. Vascular dilatation and hyperintensity on maps is less obvious compared with that shown on pretreatment maps
Discussion
Perfusion CT of the brain combines the continuous, dynamic data acquisition of a single section with the IV administration of an iodinated contrast agent bolus. This technique allows observation and quantification of the contrast agent transit through the brain. Such a method can be used to assess asymmetric changes in cerebral perfusion—ischemic effects, which result in a slow and attenuated enhancement and autoregulatory vasodilation, which yield an increased enhancement and delayed washout. Thus, CT perfusion potentially helps in evaluating the clinical significance of extracranial and intracranial vascular stenoses, in planning the need for revascularization procedures, and in predicting a patient's prognosis after a cerebral ischemia.
In the presented case of a patient with a right carotid artery stenosis, the CT perfusion technique reported changes in perfusion parameters that were expected after carotid revascularization. Before carotid stent placement, there was decreased perfusion in the ipsilateral insula (Fig 1). In the ipsilateral basal ganglia, we found delayed contrast agent clearance from the tissue (Fig 2), which is attributed to a dilation of those vessels supplying the basal ganglia compared with the normal side. The asymmetric insular findings were resolved on the scan obtained 1 day after treatment (Fig 1), indicating the technical success of the revascularization. In the right basal ganglia, there was still a subtle stenotic effect visible (Fig 2). These observed changes in the dynamic CT perfusion scans and maps suggest that dynamic CT perfusion may be a useful tool in the evaluation of patients with asymmetrical blood flow to the brain.
The technique of CT perfusion was originally described in the early 1980s (11−13). At that time, CT was limited mainly by low temporal resolution. Currently, however, newer technologies allow a temporal resolution of 1 second or less per scan, which is necessary to observe the transit of the IV-administered contrast agent through the brain.
Recent publications describe the use of contrast-enhanced helical CT to calculate fBV maps of patients with acute stroke (14). Although this is useful for patients with acute stroke and thus reduced blood volume, for patients with chronic vascular stenoses, as in the present case, the mere calculation of fBV maps does not reveal sufficient information. Patients with chronic carotid stenoses might have decreased cerebral perfusion, and thus decreased contrast enhancement, in combination with arteriolar vasodilation from autoregulation, resulting in delayed washout (15−17). Paradoxically, this physiologic compensation may lead to increased or unchanged fBV values compared with the normally perfused tissue.
Using dynamic contrast-enhanced CT perfusion, one can separate the components of perfusion and vascular compartment volume. One must ensure that the cine sequence covers the complete contrast agent transit curve. Thus, scanning has to be extended over 30 seconds. With such a protocol, abnormalities in perfusion can be detected in the wash-in phase (eg, delay and attenuation in contrast agent arrival) (Fig 1) as well as changes in the vascular volume in the washout phase (from autoregulation) (Fig 2). Both delayed enhancement (15) and an increase in blood volume have been found using MR imaging by previous investigators (16), in agreement with the CT perfusion changes in the present case.
From the acquired data, it is possible to subtract images, calculate maps based on a pixel-by-pixel analysis (Fig 3, 4), or draw regions of interest in areas that are then used to calculate the parameters of interest. In our experience, changes in contrast agent transit are sometimes too subtle to be seen on subtraction images or even maps but are, however, clear in time-course analysis of region-of-interest measurements.
The combination of CT angiography and CT perfusion can reveal the extent of collateral CBF. It has been described (14) that the combination of 3D functional CT and “perfused” blood volume can provide information regarding brain at risk for hypoperfusion. To address this further, the cine sequence could be repeated under stressful conditions, either during breathhold (18, 19) or pharmacologic intervention (20, 21), both of which have been tested with MR imaging.
Compared with MR imaging, perfusion assessment with dynamic CT has certain advantages. CT is still the primary imaging technique to exclude intracranial hemorrhage or to reveal infarcted, hypoattenuated areas or edema among patients with brain ischemia (22, 23). Helical CT scanners are much more readily available, and the acquisition is fast and reliable. As opposed to MR images, with which the relationship between the contrast agent concentration and the signal intensity is nonlinear, there is a linear relationship between the density changes on CT scans and the contrast agent accumulation, allowing more robust kinetic analysis (7). The disadvantages are that, with most CT scanners, a dynamic series to observe the whole contrast agent transit through a tissue, including wash-in and wash-out phase, can be obtained in a single section only. Future developments, such as multisection CT, will alleviate this obstacle. Other disadvantages include the patient's exposure to irradiation and contrast agent administration. To address the first concern, for the cine sequence, the tube current was reduced to 100 mA. To address the latter concern, the contrast agent administration was split into two doses. The injection of 50 mL for the cine sequence allows for a compact bolus and is still sufficient to achieve detectable increases in Hounsfield units. Additional 100 mL for the subsequent helical scan is both necessary and sufficient for calculating blood volume maps and obtaining high-quality CT angiograms. Provided that normal kidney function and hydration is maintained, the whole administered amount of 150 mL is low enough that the proposed protocol can be applied in a patient with a presumed acute stroke who might proceed to angiography after the CT study is finished (24, 25).
A general limitation of dynamic CT perfusion as well as MR perfusion studies is their qualitative result. One can compare flow parameters only between hemispheres, which might be limiting in a case of a bilateral disease. Also, the long bolus administration time might cause some variability, so that it might be difficult to generate normal values from a collective of volunteers.
In summary, dynamic CT perfusion may be a useful tool to detect brain perfusion asymmetry, which may be relevant to help plan the need for revascularization procedures, such as endarterectomy, angioplasty, stenting, and bypass surgery. The proposed protocol combines the assessment of the cerebral vasculature with CT angiography as well as the perfusion of the brain tissue, which may help tailor emergent stroke therapy. At our institution, the presented protocol is used routinely in all patients with chronic ischemia for whom a revascularization procedure is planned. Furthermore, we currently implement this technique for patients with suspected acute infarction to investigate the role of dynamic CT perfusion in confirming the diagnosis of stroke and predicting eventual brain infarct size.
Footnotes
Address reprint requests to Heidi C. Roberts, MD, Department of Radiology, Box 0628, University of California, San Francisco, 513 Parnassus Avenue, San Francisco, CA 94143.
References
- 1.Astrup J, Siesjš B, Symon L. Thresholds in cerebral ischemia: the ischemic penumbra. Stroke 1981;12:723-725 [DOI] [PubMed] [Google Scholar]
- 2.Heiss W. Experimental evidence of ischemic thresholds and functional recovery. Stroke 1992;12:1668-1672 [DOI] [PubMed] [Google Scholar]
- 3.Powers WJ, Zivin J. Magnetic resonance imaging in acute stroke: not ready for prime time. Neurology 1998;50:842-843 [DOI] [PubMed] [Google Scholar]
- 4.Kucharczyk J, Roberts T, Moseley M, Watson A. Contrast-enhanced perfusion-sensitive MR imaging in the diagnosis of cerebrovascular disorders. JMRI 1993;3:241-245 [DOI] [PubMed] [Google Scholar]
- 5.Sorensen AG, Wray SH, Weisskoff RM, et al. Functional MR of brain activity and perfusion in patients with chronic cortical stroke. AJNR Am J Neuroradiol 1995;16:1753-1762 [PMC free article] [PubMed] [Google Scholar]
- 6.Gillard JH, Hardingham CR, Kirkpatrick PJ, Antoun NM, Freer CE, Griffiths PD. Evaluation of carotid endarterectomy with sequential MR perfusion imaging: a preliminary report. AJNR Am J Neuroradiol 1998;19:1747-1752 [PMC free article] [PubMed] [Google Scholar]
- 7.Koenig M, Klotz E, Luka B, Venderink DJ, Spittler JF, Heuser L. Perfusion CT of the brain: diagnostic approach for early detection of ischemic stroke. Radiology 1998;209:85-93 [DOI] [PubMed] [Google Scholar]
- 8.Nagata K, Asano T. Functional image of dynamic computed tomography for the evaluation of cerebral hemodynamics. Stroke 1990;21:882-889 [DOI] [PubMed] [Google Scholar]
- 9.Gobbel GT, Cann CE, Iwamoto HS, Fike JR. Measurement of regional cerebral blood flow in the dog using ultrafast computed tomography: experimental validation. Stroke 1991;22:772-779 [DOI] [PubMed] [Google Scholar]
- 10.Gobbel GT, Cann CE, Fike JR. Measurement of regional cerebral blood flow using ultrafast computed tomography: theoretical aspects. Stroke 1991;22:768-771 [DOI] [PubMed] [Google Scholar]
- 11.Axel L. Cerebral blood flow determination by rapid-sequence computed tomography: theoretical analysis. Radiology 1980;137:679-686 [DOI] [PubMed] [Google Scholar]
- 12.Berninger WH, Axel L, Norman D, Napel S, Redington RW. Functional imaging of the brain using computed tomography. Radiology 1981;138:711-716 [DOI] [PubMed] [Google Scholar]
- 13.Norman D, Axel L, Berninger WH, et al. Dynamic computed tomography of the brain: techniques, data analysis, and applications. AJR Am J Roentgenol 1981;136:759-770 [DOI] [PubMed] [Google Scholar]
- 14.Hunter GJ, Hamberg LM, Ponzo JA, et al. Assessment of cerebral perfusion and arterial anatomy in hyperacute stroke with three-dimensional functional CT: early clinical results. AJNR Am J Neuroradiol 1998;19:29-37 [PMC free article] [PubMed] [Google Scholar]
- 15.Nighoghossioan N, Berthezene Y, Philippon B, Adeleine P, Froment JC, Trouillas P. Hemodynamic parameter assessment with dynamic susceptibility contrast magnetic resonance imaging in unilateral symptomatic internal carotid artery occlusion. Stroke 1996;27:474-479 [DOI] [PubMed] [Google Scholar]
- 16.Gückel FJ, Brix G, Schmiedek P, et al. Cerebrovascular reserve capacity in patients with occlusive cerebrovascular disease: assessment with dynamic susceptibility contrast-enhanced MR imaging and the acetazolamide stimulation test. Radiology 1996;201:405-412 [DOI] [PubMed] [Google Scholar]
- 17.Todd NV, Picozzi P, Crockard HA. Quantitative measurement of cerebral blood flow and cerebral blood volume after cerebral ischaemia. J Cereb Blood Flow Metab 1986;6:338-341 [DOI] [PubMed] [Google Scholar]
- 18.Kastrup A, Li TQ, Takahashi A, Glover GH, Moseley ME. Functional magnetic resonance imaging of regional cerebral blood oxygenation changes during breath holding. Stroke 1998;29:2641-2645 [DOI] [PubMed] [Google Scholar]
- 19.Li TQ, Kastrup A, Takahashi AM, Moseley ME. Functional MRI of human brain during breath holding by BOLD and FAIR techniques. Neuroimage 1999;9:243-249 [DOI] [PubMed] [Google Scholar]
- 20.Kleinschmidt A, Steinmetz H, Sitzer M, Merboldt K, Frahm J. Magnetic resonance imaging of regional cerebral blood oxygenation changes under acetazolamide in carotid occlusive disease. Stroke 1995;26:106-110 [DOI] [PubMed] [Google Scholar]
- 21.Petrella JR, DeCarli C, Dagli M, et al. Age-related vasodilatory response to acetazolamide challenge in healthy adults: a dynamic contrast-enhanced MR study. AJNR Am J Neuroradiol 1998;19:39-44 [PMC free article] [PubMed] [Google Scholar]
- 22.von Kummer R, Bozzazo L, Manelfe L. CT Diagnosis of Hemispheric Brain Infarction.. Berlin: Springer-Verlag; 1995
- 23.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017-1025 [PubMed] [Google Scholar]
- 24.Hunter JV, Kind PR. Nonionic iodinated contrast media: potential renal damage assessed with enzymuria. Radiology 1992;108:101-104 [DOI] [PubMed] [Google Scholar]
- 25.Rosovsky MA, Rusinek H, Berenstein A, Basak S, Setton A, Nelson PK. High-dose administration of nonionic contrast media: a retrospective review. Radiology 1996;200:119-122 [DOI] [PubMed] [Google Scholar]


