Abstract
We present a patient with severe middle cerebral artery occlusion who received an intra-arterial infusion of autologous bone marrow stem cells combined with Cerebrolysin IV. The patient was evaluated before and after treatment using the National Institutes of Health Stroke Scale, the Medical Research Council Muscle Scale, Modified Brunnstrom Classification, Barthel Index and modified Rankin Scale. After the therapy, the patient showed good outcome with functional as well as neurological improvements especially in terms of functional motor recovery without any side effects. Further controlled studies are needed to find possible side effects and establish net efficacy.
Keywords: Stem cell therapy, cerebrolysin, ischemic stroke
Introduction
Ischemic stroke, of all strokes, is a major cause of serious long-term disability and the therapeutic efficacy of medical treatment is usually very limited.1 In particular, large middle cerebral artery (MCA) infarction poses an increased risk of mortality (by 17%) and severe disability (by 50%) compared to other infarcts.2 One of the most encouraging innovative treatment options is restorative therapy using stem cell replacement in the injured cerebral tissues. Although evidence of the beneficial effects of stem cells in animal stroke models is growing, there is a lack of clinical data.3–5
Cerebrolysin is a neuropeptide preparation with neuroprotective and neurorestorative effects. In stroke patients with severe motor impairment, patients receiving intravenous infusions of Cerebrolysin had significantly better improvements in motor function compared with those who do not receive Cerebrolysin.6
This case report presents the good outcome of a patient with MCA occlusion after intra-arterial infusion of autologous bone marrow stem cell (BMSC) combined with Cerebrolysin .
Case report
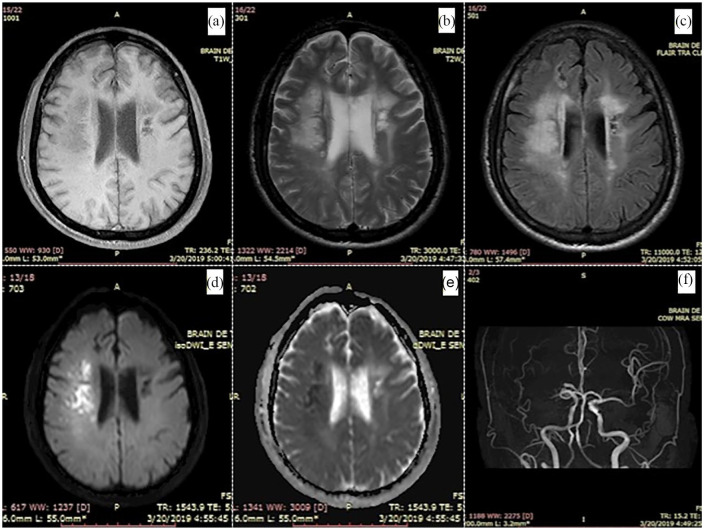
A 58-year-old man with a medical history of hypertension and hyperlipidemia was admitted to our stroke center 1 day after a sudden left-sided weakness and facial drooping. Physical examination showed clouding of consciousness, hemineglect and left-sided hemiparesis. The initial NIHSS score7 was 17. He was bedridden and required constant nursing care and attention, corresponding with modified Rankin Scale (mRS) of 5. Head magnetic resonance imaging (MRI) on day 14 after stroke onset (pre-therapy) showed a large hyperintensity lesion on diffusion-weighted imaging (DWI; Figure 1(d)) and hypointensity on the apparent diffusion coefficient (ADC) image (Figure 1(e)) in the right hemisphere consistent with an acute infarct. Magnetic resonance (MR) angiography revealed occlusion at the M1 segment of the right MCA (Figure 1(f)). Patient received medical treatment according to the American Heart Association and the American Stroke Association (AHA/ASA) stroke guideline.
Figure 1.
Head MRI at day 14 from stroke onset (pre-therapy) showed a large hyperintensity lesion on DWI (d), T2W (b), T2FLAIR (c) images and hypointensity on ADC (e), T1W (a) images in the right hemisphere. MR angiography revealed occlusion at the M1 segment of the right MCA (f).
In total, 14 days after stroke onset, the patient still showed severe disability: left-sided hemiparesis (upper extremity: MRC grade 1; lower extremity: MRC Grade 2)8,9 and there was no movement in the patient’s hands and fingers (MBC Grade 1).10,11 The patient was not able to sit on the bed and required constant nursing care (mRS 5).12 He still had unilateral neglect and some symptoms of right hemispheric damage. The patient scored 12 on the NIHSS and 10 on the Barthel Index (BI) scales. We decided to offer the patient the following treatment:
Intra-arterial infusion of 1 × 106 autologous bone marrow mononuclear cells (BMMCs), infused through the internal carotid artery, using digital subtraction angiography;
A 10-day treatment course with daily intravenous infusion of 20 mL of Cerebrolysin, repeated every month for the first 6 months after the stroke.
After infusion of BMMCs, the patient was evaluated every hour for the first 24 h for vital signs, any neurological deterioration as well as anaphylactic reactions, and then every day for the following week. The patient was discharged on day 28.
On day 45 after stroke, which corresponds to day 30 after infusion of BMMCs and initiation of Cerebrolysin treatment, the patient showed remarkable improvements, in particular, in terms of motor function recovery. Motor strength was 5/5 for both upper and lower extremities, and the patient was able to walk without assistance. He had regained motor function in his hands and fingers almost completely and was able to hold objects in his hand (MBC 5). However, certain neurological sequelae could be observed, such as facial palsy, dysarthria and neglect—corresponding to an NIHSS score of 4. The patient required help with certain daily activities, such as showering and climbing stairs, and also had cognitive deficits, corresponding to an mRS of 3 and a BI score of 75 (Table 1).
Table 1.
NIHSS, MRC grade, MBC, BI and mRS score over time.
| Scale | Baseline | Day 14 (Pre-therapy) | Day 45 | Day 105 | Day 195 |
|---|---|---|---|---|---|
| NIHSS (all items) | 17 | 12 | 4 | 3 | 3 |
| MRC grade (upper extremity) | – | 1 | 5 | 5 | 5 |
| MRC grade (lower extremity) | – | 2 | 5 | 5 | 5 |
| MBC (hand) | – | 1 | 5 | 5 | 5 |
| Barthel index | – | 10 | 75 | 90 | 90 |
| mRS | – | 5 | 3 | 2 | 2 |
On day 105, corresponding to day 90 after initiation of BMMC and Cerebrolysin treatment, further improvements were observed, especially in terms of cognitive function. The patient was able to carry out daily activities corresponding to an mRS of 2 and a BI score of 90. The patient still had some remaining signs of cognitive decline resembling mild cognitive impairment (MCI), neglect and facial paralysis. The NIHSS score was 3 (Table 1).
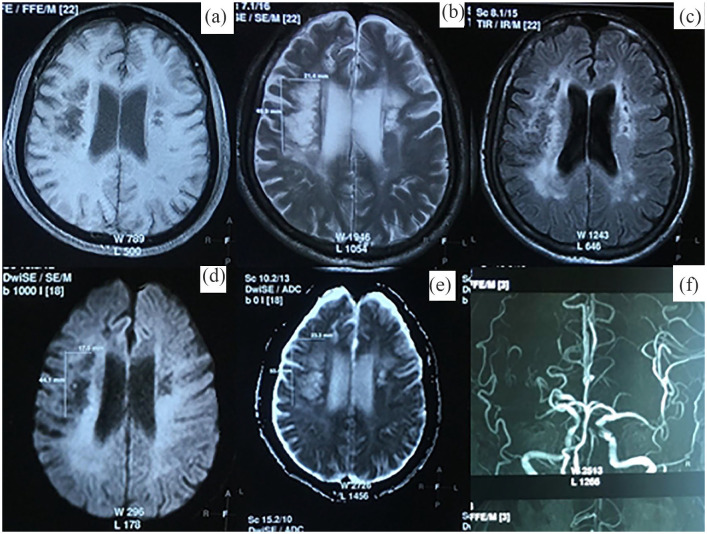
At the final examination on day 195 (day 180 after therapy), no further improvement could be observed. The patient was stable, with assessment scales remaining the same as on day 105 (NIHSS 3, mRS 2 and BI 90; Table 1). Head MRI showed a hypointense lesion on DWI and hyperintense lesion on ADC, with the dimensions of 23 × 53 × 20 mm (Figure 2), having reduced in size compared to day 14 (30 mm × 55 mm × 25 mm; Figure 1).
Figure 2.
Head MRI at day 195 from stroke onset (6 months after therapy) showed a large hyperintensity lesion on ADC (e), T2 W (b), T2FLAIR (c) images and hypointensity on DWI (d), T1 W (a) images in the right hemisphere. MR angiography revealed occlusion at the M1 segment of the right MCA (f).
During and after using this therapy, we found no adverse effects, which are related to the techniques as well as cell therapy. Study testing disclosed no safety concerns. Serial physical exams and blood testing did not disclose any significant findings.
Discussion
Normally, the prognosis of patients with a large MCA infarction is very poor. Even with the current optimal medical treatment, MCA infarction may lead to death in 17% of cases, and survivors frequently suffer from long-term severe disability (50%), especially in the motor and speech function.2 Unfortunately, the survivors have no effective treatment available other than neurorehabilitation in order to improve neurological functions. In recent years, steady progress in the clinical applications of regenerative medicine has occurred for a variety of diseases. Cell transplantation has emerged as a promising treatment for stroke recovery. Depending on this approach, we have decided to use this new therapy for patient.
In our case study, BMSCs were infused for patient at day 17 from the onset corresponding to subacute stage of cerebral infarction. We selected this time window upon revision of preclinical trials and clinical trials.5,14 Also, stroke patients are liable for deterioration in early days either by the effect of cerebral edema or hemorrhagic transformation. Finally, some patients with ischemic stroke have spontaneous recovery, therefore, we selected the subacute stage to be sure that the patient has stable deficit due to ischemic stroke.
After the therapy, the patient showed remarkable outcome. His improvements manifested most clearly in terms of motor function, especially the recovery of the motor function in the hand. It is well known that the recovery of motor function in upper extremities, particularly in the hand, is harder to achieve compared to in lower extremities due to its complex structure, specialized functions.15 Jang et al. investigated the motor outcomes in 23 patients with a complete middle cerebral artery territory infarct, about 70% of these patients were able to walk independently after 3–6 months, but no patient achieved functional hand recovery.10
As mentioned earlier, while many treatment methods have been developed, there is a lack of effective therapies that can facilitate the recovery of patients from subacute phase post-stroke, and rehabilitation is the only method that is known to contribute to the functional recovery. Several rehabilitation measures, such as robotic rehabilitation therapy and mirror therapy, have been reported to be effective in improving hand function of stroke survivors with severe sequelae.16,17 Apart from their individual impact, research shows that exercise enhances the effect of stem cells by helping the mobilization of local stem cells and encouraging angiogenesis. Hence, the concept of neuroregenerative rehabilitation therapy (NRRT) is becoming a potential research direction in the hope of finding a more effective approach to treat stroke sequelae.18
In terms of the safety of the therapy, patient was closely followed during and after the treatment (6 months), no side effect was noted. We chose autologous BMSCs for treatment because they are easily accessible through the aspiration of the bone marrow, can be isolated from patients themselves thereby bypassing the ethical problems and can easily be administered to the patients for autotransplantation. Furthermore, some studies have demonstrated the safety and advantages of this kind of stem cell.4,5 We referred to the dose of BMSCs based on a number of previous studies and the results of these studies showed that they did not find any dose-response association, raising doubt about cause and effect relationship between BMSCs and outcome.4,5 Previous research has shown that Cerebrolysin is safe and has positive effects on neurorecovery after various types of brain lesions such as ischemic stroke and brain trauma.6,19–21
Conclusion
In conclusion, intra-arterial infusion of BMSCs combined with long-term concomitant treatment of Cerebrolysin over 6 months appeared to be safe therapy without adverse effects in our case. Functional and neurological improvement, especially in terms of functional motor recovery, were initially observed in this patient who initially had a very poor prognosis due to the MCA occlusion. To corroborate our findings, larger and controlled clinical studies should be initiated to further investigate the efficacy and safety of BMSCs therapy combined with Cerebrolysin in the treatment of disability in ischemic stroke.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval to report this case was obtained from Independent Ethics Committee (IEC) of 108 Military Central hospital (APPROVAL NUMBER: 59/QĐ-BV108).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iD: Vien C Le  https://orcid.org/0000-0003-4978-4785
https://orcid.org/0000-0003-4978-4785
References
- 1. Donkor ES. Stroke in the 21(st) century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat 2018; 2018: 3238165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heinsius T, Bogousslavsky J, Van Melle G. Large infarcts in the middle cerebral artery territory etiology and outcome patterns. Neurology 1998; 50(2): 341–350. [DOI] [PubMed] [Google Scholar]
- 3. Misra V, Ritchie MM, Stone LL, et al. Stem cell therapy in ischemic stroke: role of IV and intra-arterial therapy. Neurology 2012; 79: S207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prasad K, Sharma A, Garg A, et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke 2014; 45(12): 3618–3624. [DOI] [PubMed] [Google Scholar]
- 5. Ghali AA, Yousef MK, Ragab OA, et al. Intra-arterial infusion of autologous bone marrow mononuclear stem cells in subacute ischemic stroke patients. Front Neurol 2016; 7: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang WH, Park C-H, Kim D, et al. Cerebrolysin combined with rehabilitation promotes motor recovery in patients with severe motor impairment after stroke. BMC Neurol 2016; 16: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lyden P. Using the National Institutes of Health Stroke Scale 2017; 48: 513–519. [DOI] [PubMed] [Google Scholar]
- 8. Gregson JM, Leathley MJ, Moore AP, et al. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing 2000; 29(3): 223–228. [DOI] [PubMed] [Google Scholar]
- 9. James M. Use of the medical research council muscle strength grading system in the upper extremity. J Hand Surg Am 2007; 32(2): 154–156. [DOI] [PubMed] [Google Scholar]
- 10. Jang SH, Chang MC. Motor outcomes of patients with a complete middle cerebral artery territory infarct. Neural Regeneration Research 2013; 8: 1892–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naghdi S, Ansari NN, Mansouri K, et al. A neurophysiological and clinical study of Brunnstrom recovery stages in the upper limb following stroke. Brain Inj 2010; 24(11): 1372–1378. [DOI] [PubMed] [Google Scholar]
- 12. Broderick JP, Adeoye O, Elm J. Evolution of the Modified Rankin Scale and its use in Future Stroke Trials. Stroke 2017; 48(7): 2007–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Med J 1965; 14: 61–65. [PubMed] [Google Scholar]
- 14. Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res 2003; 73: 778–786. [DOI] [PubMed] [Google Scholar]
- 15. Carbajal-Galarza M, Chinchihualpa N, Abanto-Perez S, et al. Effectiveness of technological interventions to improve upper limb motor function in people with stroke in low- and middle-income countries: protocol for a systematic review and meta-analysis, 2020, https://www.medrxiv.org/content/10.1101/2020.11.10.20209197v1.full.pdf
- 16. Wang H, Arceo R, Chen S, et al. Effectiveness of interventions to improve hand motor function in individuals with moderate to severe stroke: a systematic review protocol. BMJ Open 2019; 9: e032413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ranzani R, Lambercy O, Metzger JC, et al. Neurocognitive robot-assisted rehabilitation of hand function: a randomized control trial on motor recovery in subacute stroke. J Neuroeng Rehabil 2020; 17: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ito A, Kubo N, Liang N, et al. Regenerative rehabilitation for stroke recovery by inducing synergistic effects of cell therapy and neurorehabilitation on motor function: a narrative review of pre-clinical studies. Int J Mol Sci 2020; 21: 3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muresanu DF, Heiss W-D, Hoemberg V, et al. Cerebrolysin and recovery after stroke (CARS). Stroke 2016; 47: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muresanu DF, Florian S, Hömberg V, et al. Efficacy and safety of cerebrolysin in neurorecovery after moderate-severe traumatic brain injury: results from the CAPTAIN II trial. Neurol Sci 2020; 41(5): 1171–1181. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, Chopp M, Zhang Y, et al. Randomized controlled trial of Cerebrolysin’s effects on long-term histological outcomes and functional recovery in rats with moderate closed head injury. J Neurosurg. Epub ahead of print 6 September 2019. DOI: 10.3171/2019.6.Jns191027. [DOI] [PubMed] [Google Scholar]