Abstract
Background:
The objective of the study was to identify the factors that alter the length of hospital stay of COVID-19 patients so we have an estimate of the duration of hospitalization of patients. To achieve this, we used a time to event analysis to arrive at factors that could alter the length of hospital stay, aiding in planning additional beds for any future rise in cases.
Methods:
Information about COVID-19 patients was collected between June and August 2020. The response variable was the time from admission to discharge of patients. Cox proportional hazard model was used to identify the factors that were associated with the length of hospital stay.
Results:
A total of 730 COVID-19 patients were included, of which 675 (92.5%) recovered and 55 (7.5%) were considered to be right-censored, that is, the patient died or was discharged against medical advice. The median length of hospital stay of COVID-19 patients who were hospitalized was found to be 7 days by the Kaplan Meier curve. The covariates that prolonged the length of hospital stay were found to be abnormalities in oxygen saturation (HR = 0.446, P < .001), neutrophil-lymphocyte ratio (HR = 0.742, P = .003), levels of D-dimer (HR = 0.60, P = .002), lactate dehydrogenase (HR = 0.717, P = .002), and ferritin (HR = 0.763, P = .037). Also, patients who had more than 2 chronic diseases had a significantly longer length of stay (HR = 0.586, P = .008) compared to those with no comorbidities.
Conclusion:
Factors that are associated with prolonged length of hospital stay of patients need to be considered in planning bed strength on a contingency basis.
Keywords: COVID-19, Cox PH model, Kaplan Meier, length of stay, survival analysis
Introduction
In December 2019, COVID-19 was first identified in Wuhan, China. Since then, COVID-19 has spread to all the 7 continents including Antarctica and has created a great impact on everyone’s life.1 As of January 2021, close to 103 million cases have been reported globally, of which over 75 million people recovered and close to 2.3 million deaths have occurred.2 In India, 10.7 million confirmed cases have been reported, of which 10.3 million people recovered and over 154 428 deaths have been reported according to official figures released by the Union Ministry of Health and Family Welfare. The first COVID-19 case in India was reported on 30th January 2020.3 The most common symptoms are fever and dry cough, less common symptoms being fatigue, loss of smell and taste, myalgia, joint pain, sore throat, headache, vomiting, and diarrhea. Serious symptoms are shortness of breath and symptoms suggestive of thrombosis or ischemia such as angina, focal neurological deficits, etc. A few people are asymptomatic but they may also transmit the virus, although relatively less than symptomatic people. Everyone is at risk for getting COVID-19 if they are exposed to the virus and some may require hospitalization and intensive care. World Health Organization (WHO) stated that almost 85% of COVID-19 cases have mild to moderate symptoms and illness, 10% to 15% people have severe symptoms and out of these severe symptoms only 5% may require ICU care. According to WHO, the median time from onset to clinical recovery for mild cases is approximately 2 weeks and 3 to 6 weeks for patients with severe or critical disease.4 The incidence of COVID-19 is still rising worldwide, though better controlled in India. Hence, it is essential to study the factors influencing the length of stay so that the pathological mechanisms underlying these factors can be dealt with to give effective interventions or treatment. Since the cases have been rapidly increasing it is important to address the length of stay, by which the availability of beds in the hospital, especially in the Intensive Care Unit (ICU) can be addressed. Though clinical presentations have been analyzed with respect to recovery time, comorbidities, and certain laboratory parameters such as creatinine, Lactate dehydrogenase (LDH), Ferritin, hemoglobin, neutrophil-lymphocyte ratio (NLR), and D-dimer have not been addressed in earlier studies as a whole to model recovery time. Since there is paucity of data on these parameters in India, this study was planned to determine the recovery time of COVID-19 patients and factors associated with it.
Materials and Methods
The study was conducted at Sri Ramachandra Institute of Higher Education and Research (SRIHER), Tamil Nadu. Clinical Trial Registry-India (CTRI) registration was done and ethical clearance obtained from the Institutional Ethics Committee. Information about study participants was retrieved from hospital records. The study population was all RT-PCR proven COVID-19 positive cases who were admitted to the hospital for treatment between 21st June 2020 and 31st August 2020. Patients who were admitted for other illnesses and incidentally found to be positive were excluded. A total of 730 COVID-19 patients (males-456, females-274) were included in the study.
Data collection included age, gender and past medical history such as diabetes, hypertension, coronary artery disease (CAD), chronic kidney disease (CKD), bronchial asthma, and seizure. Clinical symptoms like fever, cough, sore throat, breathlessness, myalgia, loss of smell and taste, vomiting, and loose stool were included. Laboratory parameters such as oxygen saturation (SpO2), creatinine, lactate dehydrogenase (LDH), ferritin, hemoglobin (Hb), total leucocyte count (TC), differential count (neutrophil, lymphocyte counts, and neutrophil lymphocyte ratio-NL ratio derived), and D-dimer were collected.
Survival analysis is a form of data analysis in which the outcome variable is time till the event occurs. This time is known as the survival time. The event could be recovery, relapse, or death.
The advantage of survival analysis is that it includes the censored data which facilitates the covariates thus making the model more preferable one. The dependent variable or the response variable in this study is the time until the occurrence of an event which is length of stay (discharge from hospital) in days. Observations are censored if the event of interest does not occur, that is the patient dies or is discharged against medical advice. To adjust for the explanatory variables, the most commonly used statistical method is Cox proportional Hazard model
Hazard function hi(t) is a combination of 2 terms, the baseline hazard h0(t) and the exponential term, “xi”s are covariates and “ßi”s are the regression coefficients of the corresponding xi’s.
Statistical Analysis
Data was analyzed using the software SPSS, version 16. Survival curves were plotted using Kaplan Meier survival curve to compare the survival probability at different points of time and to compare between 2 or more groups. Log-rank test was applied to find the statistical significance of the categories of qualitative variables. The univariate Cox proportional hazard model (Cox PH model) was used to identify the factors associated with the length of hospital stay. Then multiple cox model was used to adjust for the explanatory variable. Here, only the significant factors which were associated with the length of stay in the univariate model were considered. The assumption of Cox PH model was checked using a log-log plot to determine if the hazard is constant across time for all significant explanatory variables. Statistical significance was considered if the p-value was <0.05.
Results
In this study, a total of 730 COVID-19 patients were included, of which 675(92.5%) recovered, 30(4.1%) were discharged against medical advice, and 25(3.4%) died. Totally 55(7.5%) were considered to be right censored. That is, event of interest is not exactly known.
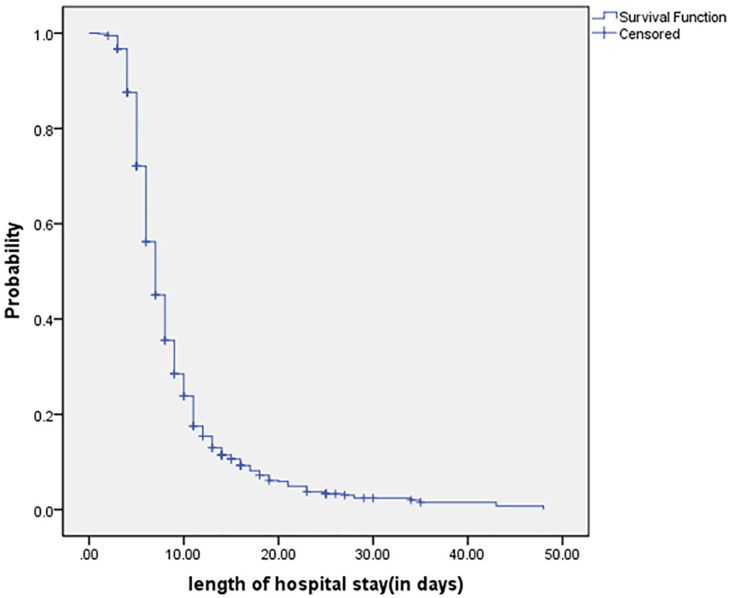
The median age was 48 years with inter quartile range of (35-61). From Table-1 it can be seen that females, younger patients, those without comorbidities, patients with oxygen saturation >95%, and those with lower values of D-dimer, ferritin, LDH, NL ratio of <3 recovered faster. As shown in Figure 1, the median length of stay (LOS) of COVID-19 patients who were hospitalized was found to be 7 days, but was longer at 17 days for patients who were more than 80 years of age. The mean time from the onset of symptoms to hospitalization was found to be 4.6 days with the standard deviation of 2.3 days and this was not associated with the recovery time (HR = 0.987, P = .407).
Table 1.
Factors Associated with the Length of Stay by Univariate Cox PH Model.
| Covariates | ß coefficient | HR (95% CI) | P-value |
|---|---|---|---|
| Gender | |||
| Male | −0.165 | 0.846 (0.724, 0.989) | .035 |
| Female (ref) | |||
| Age | |||
| ≤60 yrs (ref) | |||
| 61-80 yrs | −0.45 | 0.638 (0.529, 0.768) | <.001 |
| >80 yrs | −1.435 | 0.238 (0.113, 0.503) | <.001 |
| Fever | |||
| Yes | −0.004 | 0.996 (0.805, 1.232) | .969 |
| No (ref) | |||
| Body pain | |||
| Yes | −0.315 | 0.730 (0.449, 1.186) | .204 |
| No (ref) | |||
| Cough | |||
| Yes | −0.614 | 0.541 (0.461, 0.635) | <.001 |
| No (ref) | |||
| Breathlessness | |||
| Yes | −0.782 | 0.457 (0.383, 0.546) | <.001 |
| No (ref) | |||
| Headache | |||
| Yes | 0.031 | 1.031 (0.667, 1.594) | .889 |
| No (ref) | |||
| Loose stool | |||
| Yes | −0.336 | 0.715 (0.428, 1.194) | .200 |
| No (ref) | |||
| Myalgia | |||
| Yes | 0.089 | 1.094 (0.820, 1.459) | .543 |
| No (ref) | |||
| Sore throat | |||
| Yes | −0.744 | 0.475 (0.406, 0.556) | <.001 |
| No (ref) | |||
| Vomit | |||
| Yes | −1.071 | 0.343 (0.163, 0.723) | .005 |
| No (ref) | |||
| Loss of smell/taste | |||
| Yes | 0.472 | 1.603 (1.102, 2.332) | .014 |
| No (ref) | |||
| Diabetes mellitus | |||
| Yes | −0.395 | 0.674 (0.576, 0.789) | <.001 |
| No (ref) | |||
| Hypertension | |||
| Yes | −0.375 | 0.687 (0.578, 0.818) | <.001 |
| No (ref) | |||
| Chronic kidney disease | |||
| Yes | −0.578 | 0.561 (0.346, 0.909) | .019 |
| No (ref) | |||
| Coronary artery disease | |||
| Yes | −0.918 | 0.399 (0.279, 0.572) | <.001 |
| No (ref) | |||
| Bronchial asthma | |||
| Yes | −0.13 | 0.878 (0.579, 1.331) | .540 |
| No (ref) | |||
| Seizure | |||
| Yes | −0.039 | 0.961 (0.456, 2.026) | .917 |
| No (ref) | |||
| Comorbidities | |||
| No comorbidities (ref) | |||
| 1-2 comorbidities | −0.404 | 0.667 (0.570, 0.781) | <.001 |
| ≥3 comorbidities | −0.902 | 0.406 (0.280, 0.587) | <.001 |
| Hemoglobin (g/dL) | |||
| ≥12 (ref) | −0.154 | 0.857 (0.72, 1.021) | .857 |
| <12 | |||
| Lactate dehydrogenase (LDH) (IU/L) | |||
| ≤300 (ref) | −0.825 | 0.438 (0.367, 0.523) | <.001 |
| >300 | |||
| Creatinine (mg/dL) | |||
| ≤1.14 (ref) | −0.721 | 0.486 (0.323, 0.732) | .001 |
| >1.4 | |||
| D-dimer (µg/mL) | |||
| ≤0.55 (ref) | −0.511 | 0.600 (0.507, 0.709) | <.001 |
| >0.55 | |||
| Neutrophil-Lymphocyte (NL) ratio | |||
| ≤3 (ref) | −0.828 | 0.437 (0.369, 0.517) | <.001 |
| >3 | |||
| Oxygen saturation (SpO2) (%) | |||
| ≥95 (ref) | −1.165 | 0.312 (0.258, 0.377) | <.001 |
| <95 | |||
| Ferritin (ng/ml) | |||
| 25-200 (ref) | −0.673 | 0.51 (0.402, 0.648) | <.001 |
| <25 or >200 | |||
| Total leucocyte count (TC) (cells/cu. mm) | |||
| 4000-11 000 (ref) | −0.34 | 0.712 (0.586, 0.864) | .001 |
| <4000 or >11 000 | |||
Abbreviations: ref, reference category; HR, hazard ratio.
Figure 1.
Overall survival in COVID-19 patients by Kaplan Meier curve.
In multiple cox regression model, none of the symptoms or gender were significantly associated with LOS. Factors associated with the LOS were SpO2, ferritin, NL ratio, D-Dimer, LDH, and patients with more than 2 chronic comorbid conditions, as shown in Table 2. The single factor that most significantly influenced and prolonged length of stay was SpO2. Patients who had SpO2 level <95% had longer length of stay compared to those with SpO2 level ≥95% [HR = 0.446; 95% CI(0.358,0.555); P ≤ .001]. There was no significant difference in the length of stay between the patients those who did not have any comorbidities and those with 1 or 2 comorbidities but, those who had more than 2 comorbidities had longer length of stay when compared to those with no comorbidities [HR = 0.586; 95%CI(0.393,0.873); P = .008]. Patients with D-dimer (>0.55), the rate of discharge decreased by 40% than compared to patients with D-dimer (≤0.55) [HR = 0.600; 95% CI (0.507, 0.709); P = .002]
Table 2.
Multivariable Cox-PH Model for COVID-19 Patients.
| Covariates | ß coefficient | HR (95% CI) | P-value |
|---|---|---|---|
| Comorbidities | |||
| No comorbidities | |||
| 1-2 comorbidities | −0.073 | 0.929 (0.787, 1.106) | 0.409 |
| ≥3 comorbidities | −0.534 | 0.586 (0.393, 0.873) | 0.008 |
| Lactate dehydrogenase (LDH) (IU/L) | |||
| ≤300 (ref) | −0.333 | 0.717 (0.583, 0.882) | .002 |
| >300 | |||
| D-dimer (µg/mL) | |||
| ≤0.55 (ref) | −0.288 | 0.600 (0.507, 0.709) | .002 |
| >0.55 | |||
| Neutrophil-Lymphocyte (NL) ratio | |||
| ≤3 (ref) | −0.298 | 0.742 (0.610, 0.902) | .003 |
| >3 | |||
| Oxygen saturation (SpO2) (%) | |||
| ≥95 (ref) | −0.808 | 0.446 (0.358, 0.555) | <.001 |
| <95 | |||
| Ferritin (ng/ml) | |||
| 25-200 (ref) | −0.271 | 0.763 (0.592, 0.984) | .037 |
| <25 or >200 | |||
HR<1 indicates increased duration of hospital stay.
The Cox PH model is written as
Discussion
There have been many studies detailing the prognostic factors for COVID- 19, but there is paucity of data when it comes to duration of hospitalization in relation to these prognostic factors. It has been reported that presence of multiple comorbidities, abnormal D-dimer levels, NLR, oxygen saturation, symptoms such as breathlessness are associated with poor prognosis.5-8 We have come to a phase in this pandemic where, though we haven’t yet elucidated a “cure” for this disease, we have a few drugs which have been shown to be effective at least when used early in the disease (Ivermectin, HCQ) and some such as Remdesevir, biologicals, convalescent plasma which are reserved for the sick.9 Going by the pathogenesis and the fact that abnormal coagulation parameters have consistently been found to be bad prognosticators, anticoagulant therapy in the form of VTE prophylaxis has also helped improve survival.10 Steroids are also used to minimize the inflammation associated with COVID-19.11 With so many advancements in the understanding of the disease, the current challenge is its rapid spread causing large peaks and sudden surges in the number of people infected and thus an unfulfillable demand for hospital beds wreaking havoc in many countries across the globe.12
In our study we have seen that the median LOS was 7 days overall and 17 days for those older than 80 years. Most important factors that influenced the LOS were oxygen saturation, presence of more than 2 comorbidities and certain laboratory parameters such as LDH, ferritin, D-dimer, neutrophil-lymphocyte ratio. The death rate of COVID-19 patients in our study was observed to be low when compared to other countries, but higher (3.4%) when compared to the national average which is approximately 1.44%.3 probably because owing to the fact that we are a tertiary care hospital in the state capital and receive sicker cases.
In early studies from India, the mean length of hospital stay was seen to be as long as 17 days, IQR 15 to 20 days (where LOS was influenced significantly only by the presence of SARI or a travel history)13 to 24 days (16-34 days at 95% CI; the length of hospital stay in this study was influenced only by gender and age).14 A study done in Belgium reported that the length of stay ranged from 3 to 10.4 days and it significantly increased with the age of the patient.15 A study which was done among 538 confirmed patients between January and March 2020 in China found that the median hospital stay was 19 days with interquartile range of 14 to 23 days and it was influenced by age, serious illness, and density of health care workers.16 In a study from Vietnam on 251 patients, the median duration of hospitalization was seen to be 16 days and they found that age, residence of the patient, and the source of infection influenced the LOS significantly.17 In an unpublished study from Singapore on 187 patients, the mean length of hospital stay was 9.40 ± 7.17 days and age and nationality of the patient were found to have significant effects on the length of hospital stay.18 In a systematic review of COVID hospitalizations to assess the length of hospital stay by Rees et al19 which analyzed 52 studies from China and the rest of the world between 24th December 2019 and 16th April 2020, it was found that the length of hospital stay was much longer for patients in China when compared to the rest of the world [14 days (Inter Quartile Range (IQR) 10-19) for China and 5 days (IQR3-9) for the rest of the world] which is probably attributable to the fact that China was the first to face the pandemic and the rest of the world learnt from them.
As can be seen, there have not been many studies analyzing the factors affecting hospital LOS which could help us in making contingency plans to build infrastructure during the pandemic.
Limitations
We have not studied the impact of treatment modalities on the length of stay as it will be too elaborate from a contingency planning point of view. Also, data is limited to our center alone.
Conclusion
In this current study we have moved beyond the usual descriptive statistics to survival analysis which will give a rough estimate of the duration of hospital stay of the COVID-19 patients. The only means to prevent spread of COVID-19 are universal masking, social distancing, hand hygiene, and vaccination but getting even 50% compliance in community driven initiatives is a far-fetched dream. Hence, we may have to wait for vaccination as the next best option. But, at the rate at which new strains are emerging in this small, very connected world, the need of the hour is to devise a ready planner such that if need be, more infrastructure can be created or readied in time so that patients don’t suffer. The estimated length of stay of these patients is needed so that we can model bed-occupancy and make contingency plans. This could also aid researchers in the field of artificial intelligence to create algorithms to this effect. We hope to see more such studies from various parts of the world soon so that we can manage the growing number of patients more efficiently.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Gayathri Thiruvengadam  https://orcid.org/0000-0003-2753-0717
https://orcid.org/0000-0003-2753-0717
References
- 1. Carolyn Crist. Antarctica Reports First COVID-19-19 Outbreak. WebMD Health News Brief. December 24, 2020. Accessed January 1, 2021. https://www.webmd.com/lung/news/20201224/antarctica-reports-first-covid-19-outbreak
- 2. Worldometer. COVID-19 Coronavirus Pandemic. Coronavirus Update (Live). Accessed February 1, 2021. https://www.worldometers.info/coronavirus
- 3. Ministry of Health and Family welfare. COVID-19 Statewise Status. Accessed February 1, 2021. https://www.mohfw.gov.in/
- 4. World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). February 28, 2020. Accessed November 20, 2021. https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19)
- 5. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salinas-Escudero G, Carrillo-Vega MF, Granados-García V, et al. A survival analysis of COVID-19 in the Mexican population. BMC Public Health. 2020;20:1616. doi: 10.1186/s12889-020-09721-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yanez ND, Weiss NS, Romand JA, et al. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20:1742. doi: 10.1186/s12889-020-09826-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu S, Xue L, Legido-Quigley H, et al. Understanding factors influencing the length of hospital stay among nonsevere COVID-19 patients: a retrospective cohort study in a Fangcang shelter hospital. PLoS One. 2020;15:e0240959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. U.S Food and Drug Administration. Emergency Use Authorization. Coronavirus Disease 2019 (COVID-19) EUA Information. Accessed on December 20, 2020. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs [PubMed]
- 10. NIH. COVID-19 Treatment Guidelines. Antithrombotic Therapy in Patients with COVID-19. Updated December 17, 2020. Accessed December 20, 2020. https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/antithrombotic-therapy/
- 11. Mishra GP, Mulani J. Corticosteroids for COVID-19: the search for an optimum duration of therapy. Lancet Respir Med. 2021;9:e8. doi: 10.1016/S2213-2600(20)30530-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du RH, Liu LM, Yin, et al. Hospitalization and critical care of 109 decedents with COVID-19 Pneumonia in Wuhan, China. Ann Am Thorac Soc. 2020;17:839-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mishra V, Burma AD, Das SK, Parivallal MB, Amudhan S, Rao GN. COVID-19-hospitalized patients in Karnataka: survival and stay characteristics. Indian J Public Health. 2020;64:S221-S224. [DOI] [PubMed] [Google Scholar]
- 14. Barman MP, Rahman T, Bora K, Borgohain C. COVID-19 pandemic and its recovery time of patients in India: a pilot study. Diabetes Metab Syndr. 2020;14:1205-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faes C, Abrams S, Van Beckhoven D, Meyfroidt G, Vlieghe E, Hens N; Belgian Collaborative Group on COVID-19 Hospital Surveillance. Time between symptom onset, hospitalisation and recovery or death: statistical analysis of Belgian COVID-19 patients. Int J Environ Res Public Health. 2020;17:7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji JS, Liu Y, Liu R, et al. Survival analysis of hospital length of stay of novel coronavirus (COVID-19) pneumonia patients in Sichuan, China. medRxiv. 2020. Accessed November 20, 2021. https://www.medrxiv.org/content/10.1101/2020.04.07.20057299v1
- 17. Thai PQ, Toan DTT, Son DT, et al. Factors associated with the duration of hospitalisation among COVID-19 patients in Vietnam: a survival analysis. Epidemiol Infect. 2020;148: e114. doi: 10.1017/S0950268820001259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mollazehi M, Mollazehi M, Abdel-Salam AS. Modeling survival time to recovery from COVID-19: a case study on Singapore. Research square. 2020, Accessed November 20, 2021. https://www.researchsquare.com/article/rs-18600/v2
- 19. Rees EM, Nightingale ES, Jafari Y, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18:270. doi: 10.1186/s12916-020-01726-3 [DOI] [PMC free article] [PubMed] [Google Scholar]