Abstract
BACKGROUND AND PURPOSE: Early thrombolytic therapy is encouraged in hyperacute stroke, although this might result in delayed reperfusion injury. Our purpose was to investigate the serial changes in cerebral perfusion following transient ischemia by means of MR imaging and to correlate them with the histologic findings obtained by using the TdT-mediated dUTP-biotin nick-end labeling (TUNEL) assay.
METHODS: One-hour transient occlusion of the middle cerebral artery was produced in 10 cats. Serial perfusion-weighted MR imaging was performed for 3 days after reperfusion. The reperfusion characteristics in each region of the brain were classified into four groups according to the serial perfusion MR imaging status: normal perfusion (N), continuous hyperperfusion (I), early hyperperfusion and gradual decrease (II), and persistent hypoperfusion (III). After the last imaging session, a specimen was obtained, and TUNEL staining was performed. TUNEL-positive cells were counted under a high-power-field (HPF) light microscope (×200). The degree of TUNEL positivity was compared among the four reperfusion groups classified on the basis of serial perfusion-weighted imaging findings.
RESULTS: Group N had 16.8 ± 5.1 TUNEL-positive cells per HPF. Groups I and II had a statistically significant increase in TUNEL positivity (39.5 ± 13.4 and 43.6 ± 16.7 cells per HPF; P < .01, one-way analysis of variance), whereas group III had a statistically insignificant increase in TUNEL positivity (23.3 ± 6.9 cells per HPF).
CONCLUSION: Reperfusion followed by hyperperfusion induced cellular damage, although the initial MR imaging findings were normal. The inclusion of anti-reperfusion injury therapy should be considered in thrombolytic treatment.
Perfusion-weighted imaging can be used to ascertain local hemodynamic status and to obtain detailed anatomic information superior to that gleaned from other scintigraphic studies; the clinical usefulness of perfusion-weighted imaging has been well documented (1, 2). The temporal evolution of transient focal brain ischemia was previously investigated in an animal model by means of perfusion-weighted imaging (3–5). The results showed prompt restoration of blood flow or hyperperfusion after a transient cerebral ischemia.
Alone or in conjunction with other treatments, aggressive therapies such as intravenous intra-arterial thrombolysis have proved to be effective in treating hyperacute cerebral infarcts (6, 7). These treatments can be used to salvage the peri-infarct ischemic zone if they are administered within 3–6 hours of the ictus.
In the case in which the occluded vessels are recanalized, reperfusion hyperemia usually occurs and is depicted with positron emission tomography or perfusion-weighted imaging (8–10). This hyperemia results from the loss of autoregulation, the release of vasodilatory substances, and the process of neovascularization (11). Reperfusion hyperemia can save the ischemic tissue by rapidly restoring cerebral blood flow, but delayed reperfusion injury may occur because of oxidants or free radical damage (8).
Microscopic cellular damage can occur in the reperfused brain. Apoptosis, or programmed cell death, may play an important role in ischemia. It can be triggered by a variety of mechanisms, including glutamatergic excitotoxicity, free radical damage, cytokine effects, and inflammatory injury (12–13). In the transient ischemic model, reperfused brain tissue expresses various degrees of positivity for apoptosis (14); however, the exact relationship between perfusion changes and cellular damage has not yet been elucidated. The purpose of this study was to observe the gradual changes of focal cerebral ischemia after reperfusion revealed by perfusion-weighted imaging and to investigate their relationship with histopathologic alterations by using a feline model and TdT-mediated dUTP-biotin nick-end labeling (TUNEL) staining.
Methods
Animal Preparation
The animal experiments were performed in accordance with a protocol approved by the Committee for the Care and Use of Laboratory Animals at the Yonsei University College of Medicine, Seoul, Korea. Overall, 10 cats weighing 3.5–4.0 kg were anesthetized by using 5 mg/kg ketamine chloride and 2 mg/kg xylazine hydrochloride. Two more cats were used as sham controls.
During the operations, the cats’ rectal temperature was maintained at 37°C by using a heating pad. Oxygen saturation was maintained at higher than 92%. The left brachial vein and the right femoral artery were cannulated for the injection of fluid and for physiologic monitoring. The left middle cerebral artery (MCA) was exposed by means of a transorbital approach, and occluded with a microvascular clamp for a 0.4–1.0-mm vessel (Acland clamp; S&T Microlab AG, Rheinfall, Switzerland). One hour after vascular clamp placement, the clips were released and the craniotomy site was sealed with bone wax. For the control cats, only enucleation-sphenoid craniotomy was performed, without induction of transient cerebral ischemia.
Image Acquisition and Data Analysis
The animals were transferred to the MR unit (Intera, Philips Medical Systems, Best, the Netherlands). The first MR imaging session was performed 1 hour after reperfusion, followed by serial MR imaging 24 and 48 hours later. We obtained a coronal image perpendicular to a theoretical line drawn from the anterior commissure to the posterior commissure; it transected the bilateral striatum, which corresponded to the histologic sections. We obtained fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted images and then performed perfusion-weighted imaging (gradient-echo echo-planar imaging, TR/TE=1500/40, 128 × 128 matrix) after injecting a double dose of gadopentetate dimeglumine (Magnevist; Schering AG, Erlangen, Germany). Overall, 40 phase images were obtained for each section and processed by using commercially available postprocessing software (Easyvision, Philips Medical Systems). The relative cerebral blood volume (rCBV) was calculated.
To determine the evolution of the ischemic brain tissue in accordance with the perfusion-weighted imaging findings, five brain regions were investigated in each cat, giving a total of 50 areas in the 10 cats. The five regions were the superior and inferior frontal gyri, the superior and inferior temporal gyri, and the striatum. rCBV values were measured in each region, and the results were divided into four groups according to the appearance of perfusion changes: normal perfusion (N); continuous hyperperfusion, early hyperperfusion, or late normal perfusion (I); initial reperfusion hyperemia and gradual depletion of rCBV (II); and persistent hypoperfusion during the experiment (III) (Fig 1). rCBV increasing or decreasing by more than 20% compared with the value on the contralateral normal side was regarded as hyperperfusion or hypoperfusion, respectively. Statistical analysis was performed by using one-way analysis of variance to compare differences in the number of TUNEL-positive cells among the four groups and to perform the Tukey honestly significant difference (HSD) analysis for the four groups.
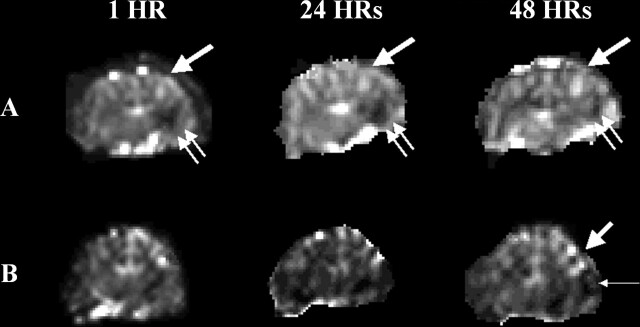
Fig 1.
Postreperfusion pattern after recanalization in the MCA occlusion model. Persistent hyperemia (type I hyperperfusion) is observed mainly in the frontal and superior temporal lobes in cats A and B (thick single arrows). Persistent hypoperfusion (type III reperfusion) is seen in the inferior temporal lobe in cat A (double arrows), and early hyperperfusion followed by later hypoperfusion (type II reperfusion) is seen in cat B (thin single arrow).
In Situ Detection of TUNEL-Positive Cells
Paraffin-embedded sections were deparaffinized by washing them twice with xylene for 5 minutes. The sections were then washed sequentially in 100%, 95%, and 75% ethanol before being incubated with 20 mg/mL proteinase K (Sigma, St Louis, MO) for 5 minutes to strip off the nuclear proteins. TUNEL was accomplished by using the Apoptag in situ kit (Roche Diagnostics, Mannheim, Germany). After the sections were immersed in an equilibration buffer for 10 minutes, they were incubated with TdT and dUTP-digoxigenin in a humidified chamber at 37°C for 1 hour, and then incubated in a stop-wash buffer at 37°C for 30 minutes to stop the reaction. The sections were then washed with phosphate buffer solution once before they were incubated in anti-digoxigenin peroxidase solution for 30 minutes. They were colorized with diaminobenzidine H2O2 solution (0.2 mg/mL tetrachloride and 0.005% H2O2 in 50 mmol/L Tris-HCl buffer) and then counterstained with methyl green. The control sections were treated similarly, but they were incubated without TdT enzyme, dUTP-digoxigenin, and anti-digoxigenin antibody. TUNEL-positive cells were counted from more than 10 areas and averaged in each anatomic region, as described before, by using high-power-field (HPF, ×200) light microscopy.
Results
Physiologic parameters, including the body temperature, blood pressure, and arterial oxygen saturation level, were within normal ranges and remained stable throughout the operation and imaging procedures. On FLAIR images, focal cerebral ischemia was observed in the striatum and cerebral cortex, mainly in the temporal lobes or some parts of the frontal lobes or both; however, individual and regional variations were large. On diffusion-weighted images, the initially hyperintense areas never returned to normal, except in one cat that had temporal reversibility in the striatum on day 1 of the imaging; however, this appeared as an infarct on the final images (Fig 2).
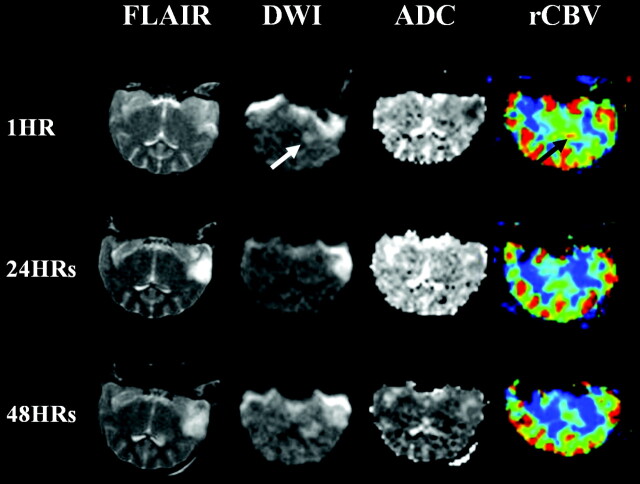
Fig 2.
Serial changes in the reperfused brain. Initial diffusion-weighted image shows a hyperintense lesion in the striatum, with temporal reversal on the image taken at 24 hours. The lesion becomes a wider area of infarction on diffusion- (white arrow) and T2-weighted images. rCBV maps show hyperperfusion in the striatum, which were shown to normalize on later images (black arrow).
Group N normal perfusion was observed mainly in the frontal gyrus. Type I reperfusion (early hyperperfusion–late normoperfusion and hyperperfusion) was widely seen in all regions, whereas type II (early hyperperfusion–late hypoperfusion) and type III (persistent hypoperfusion) reperfusion were seen mainly in the temporal cortex and striatum. The features of reperfusion varied by case, and more than one reperfusion pattern was observed in each cat. The areas of persistent hyperperfusion showed mild hyperintensity on the initial FLAIR images. Diffusion-weighted imaging did not clearly depict these areas of persistent hyperperfusion, although they demonstrated markedly increased rCBV, and visible red staining was observed after TUNEL processing (Fig 3).
Fig 3.
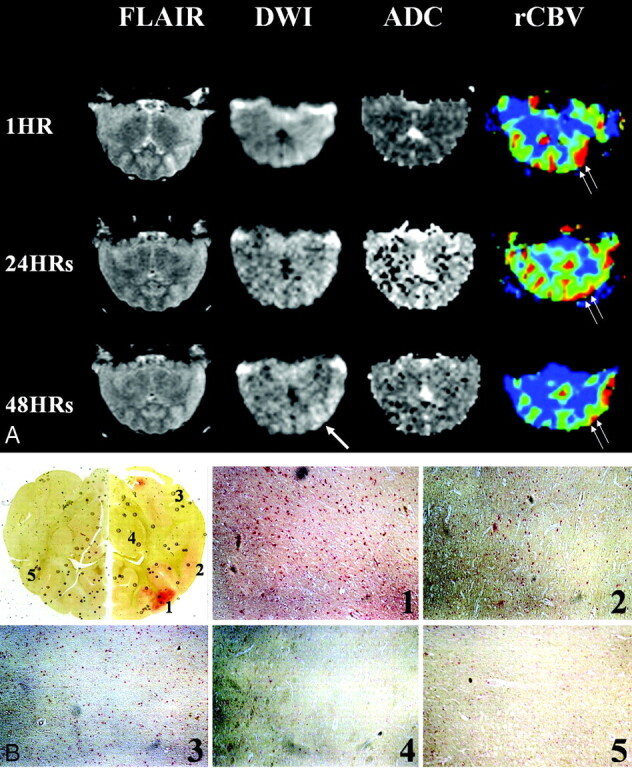
Findings in the area of persistent hyperperfusion.
A, Serial FLAIR, diffusion-weighted, and perfusion-weighted images show persistent hyperperfusion in the frontal and temporal cortices. Increased rCBV is most prominent the inferior frontal regions (double arrows). Initial FLAIR image shows cortical swelling; however, diffusion-weighted imaging does not depict abnormalities. The last diffusion-weighted images show gradually increasing signal intensity in the affected hemispheric cortex (single arrow), where persistent hyperperfusion is still observed.
B, Light photomicrographs showTUNEL staining. Red-stained areas are not hemorrhagic foci, but highly TUNEL-positive lesions coinciding with areas of hyperperfusion on perfusion-weighted images. HPF images (original magnification ×200) show multiple TUNEL-positive cells consisting of neurons and astrocytes. Density of positive cells is higher in affected frontotemporal regions than on the normal side. Red cells are also seen in the striatum that was subjected to normal perfusion and even in the contralateral cortex, but the number was less than that for the lesions. (1 indicates inferior frontal gyrus; 2, superior temporal gyrus; 3, inferior temporal gyrus; 4, striatum; and 5, normal contrlateral cortex.)
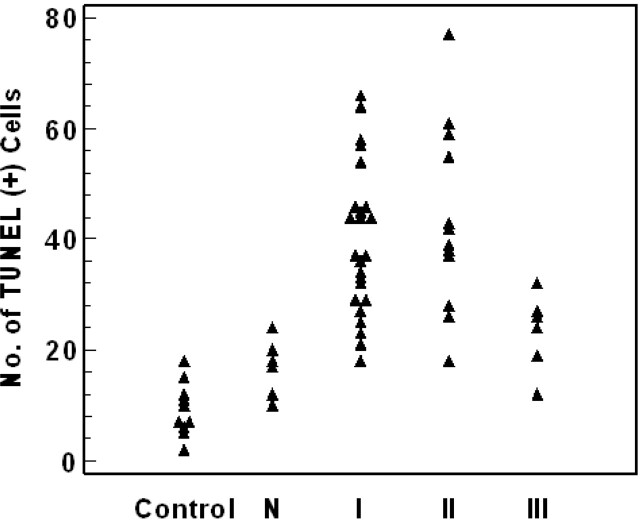
In sham-control cats, most parts of brain showed TUNEL negativity; however, some areas in the temporal lobe showed 4–12 TUNEL-positive cells in one cat, even on the normal contralateral side and in the area of normal rCBV; cell counts were 9.3 ± 4.8 and 16.8 ± 5.1 per ×200 HPF. However, significantly increased TUNEL-positive cell counts were demonstrated in areas of type I and type II reperfusion, with values of 39.5 ± 13.4 and 43.6 ± 16.7 per ×200 HPF, respectively. In the area of type III reperfusion, TUNEL positivity was lower than that of the other types of reperfusion, with a cell count of 23.3 ± 6.9 per ×200 HPF. Statistical analysis revealed significantly increased TUNEL positivity in the areas of type I or type II reperfusion, compared with the area of normal perfusion and contralateral side (P < .01; one-way analysis of variance, Tukey HSD test) (Fig 4).
Fig 4.
TUNEL-positive cell counts for each region and reperfusion patterns. Type I and II reperfusion had significantly increased TUNEL positivity, whereas type III reperfusion had only slightly increased positivity.
Discussion
We produced a model of transient MCA occlusion and demonstrated the existence of hyperperfusion after recanalization of the occluded vessels by means of perfusion-weighted imaging. We also demonstrated the occurrence of cellular changes in the area of hyperperfusion by means of the TUNEL assay. TUNEL positivity does not necessarily mean that the apoptosis pathway has been triggered, but it is an indicator of nonspecific cellular damage, including necrosis. Therefore, the results only suggest that cellular damage occurred, and some results might have been related to triggering of the apoptotic cascade. Such findings were more prominent in persistently hyperemic areas than in the initially infarcted zones, where necrosis was apparent. Therefore, we can conclude that persistent hyperperfusion during the thrombolytic period can induce cellular damage, even if the regions are not yet necrotic or infarcted.
Reperfusion and its harmful effects have been documented previously (15). Reperfusion in the form of hyperperfusion induces the secretion of strong vasodilatory substances such as potassium, bradykinin, adenosine, arachidonate, or oxygen free radicals (11), and these substances cause endothelia injury. Aggregated inflammatory cells secrete cytotoxic substances and cytokines, which eventually result in neuronal damage. Therefore, as our perfusion findings and associated cellular changes revealed, it is important to perform an imaging study after recanalizing an occluded vessel, because different hemodynamics occur after reperfusion, and these must be considered to predict the final outcome. Furthermore, these results support the rationale for early anti-reperfusion therapy in thrombolytic cases.
Diffusion abnormalities were observed in all cases, but these abnormalities were diverse. As described before, persistent hyperperfusion eventually resulted in infarction, and apparent diffusion coefficients (ADCs) also decreased in the serial images. One case had a reversible change in ADC in the striatum; however, the area became the final infarct zone on later images; this was well correlated with previous studies, which revealed a secondary decline in ADC values after reperfusion (16). Li et al (17) also proved the occurrence of irreversible cellular damage in areas of initial diffusion abnormality, although these areas showed reversibility after reperfusion. Therefore, in the clinical setting for thrombolytic therapy, combined interpretation of both diffusion- and perfusion-weighted imaging is needed, because the initial diffusion abnormality does not fully represent the status of ischemia and cannot be used to predict the final infarct.
In many hospitals, rapid restoration of cerebral perfusion is encouraged because it is believed to be beneficial in salvaging peri-infarct ischemic tissue (eg, ischemic penumbra) (7, 8). This tissue is defined as that with reduced energy metabolism and function but maintained function and morphologic mechanisms (18) that is salvageable with early reperfusion. Our results, however, strongly suggest that reperfusion may induce harmful effects in acute stroke, at least to some extent.
Previous studies in the rodent model revealed ischemic changes in the striatum within 15 minutes after the induction of ischemia, as well as frank infarction in the striatum and selective neuronal death in the cortex within 30 minutes. Irreversible damage can occur in the cerebral cortex if occlusion persists for more than 60 minutes (19–22). MCA occlusion time was 60 minutes in our experiment, because we attempted to induce a variety of ischemic injuries ranging from reversible zones to infarction. The results showed various degrees of ischemia or infarction in the frontotemporal lobes and striatum. The striatum and inferior temporal lobe, which showed overt infarction in many cases, demonstrated gradual or persistent depletion of rCBV (type III reperfusion), whereas the frontal and superior frontal lobes showed an initial increase in rCBV (type I reperfusion).
We determined that the initial hemodynamic status obtained from perfusion or diffusion studies cannot be used to predict the final infarct in the case of reperfusion, because the postreperfusion hemodynamics differ from those of the prereperfusion period. Therefore, a postreperfusion evaluation of changes observed in the imaging pattern is suitable for assessing the evolution of ischemic stroke.
This study has some limitations. First, the absolute quantification method is still not available in our institution, and we therefore used only the relative perfusion parameter, rCBV, and the temporal indicator, TTP. rCBV and TTP do not perfectly represent the cerebral perfusion status, but many centers use rCBV and TTP in the clinical field, because they are easily calculated and available by using commercial programs. Nevertheless, a future study correlating the changes of absolute cerebral blood flow values and histologic alterations is required.
We found TUNEL-positive cells in areas of normal perfusion, as well as on the normal contralateral side. Furthermore, one of the sham-control animals had minimal TUNEL-positive cells in the temporal lobe adjacent to sphenoid bone in which craniotomy and dural manipulation was performed. This phenomenon of false-positive cells is a characteristic of the cell detection kit or resulted from the environment of the laboratory process. It also results from the surgical procedure itself, spillage of CSF, changes in intracranial pressure, and dissection of the dura and arachnoid. However, all specimens were processed on the same day and with the same procedure, and we measured only the statistical increase or decrease in cellular damage. Therefore, the reliability of our result should not be challenged, although some false-positive cells were found in normal cortex.
The most important consideration is that cellular damage results not only from hyperperfusion injury but also from the initial ischemic insult. Actually, it is very difficult to make a pure hyperperfusion model of transient ischemia in vivo. Initial ischemic insult must have played a role in triggering cascades of cellular damage; this might have been a technical limitation of this study.
Conclusion
Early recanalization helps the recovery of ischemic injured brain tissue. However, in some cases, early recanalization is harmful because of the reperfusion hyperemia related to delayed cellular injury. Three perfusion patterns were observed after recanalization of the occluded vessels, and various kinds and extents of cellular damage were observed in all cases. If early recanalization is applied in the clinical field, anti-reperfusion injury treatment, such as hypothermia or the injection of anti-apoptotic material, must be considered to rescue more tissue at risk for ischemia.
Footnotes
Supported by a grant from the Korea Health 21 R&D Project (02-PJ1-PG3–21301–0013), Ministry of Health and Welfare, Korea.
References
- 1.Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamicaly weighted echo-planar MR imaging. Radiology 1996;199:390–401 [DOI] [PubMed] [Google Scholar]
- 2.Rordorf G, Koroshetz WJ, Copen WA, et al. Regional ischemia and ischemic injury in patients with acute middle cerebral artery stroke as defined by early diffusion-weighted and perfusion-weighted MRI. Stroke 1998;29:939–943 [DOI] [PubMed] [Google Scholar]
- 3.Hamberg LM, Macfarlane R, Tasdemiroglu E, et al. Measurement of cerebrovascular changes in cats after transient ischemia using dynamic magnetic resonance imaging. Stroke 1993;24:444–451 [DOI] [PubMed] [Google Scholar]
- 4.Lee SK, Kim DI, Jeong EK, Yoon PH, Cha SH, Lee JH. Temporal changes in reversible cerebral ischemia on perfusion- and diffusion-weighted magnetic resonance imaging: the value of relative cerebral blood volume maps CBV maps. Neuroradiology 2002;44:103–108 [DOI] [PubMed] [Google Scholar]
- 5.Li F, Silva MD, Sotak CH, Fisher M. Temporal evolution of ischemic injury evaluated with diffusion-, perfusion-, and T2-weighted MRI. Neurology 2000;54:689–696 [DOI] [PubMed] [Google Scholar]
- 6.Gonner F, Remonda L, Mattle H, et al. Local intra-arterial thrombolysis in acute ischemic stroke. Stroke 1998;29:1894–1900 [DOI] [PubMed] [Google Scholar]
- 7.Lewandowski CA, Frankel M, Tomsick TA, et al. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke 1999;30:2598–2605 [DOI] [PubMed] [Google Scholar]
- 8.Marchal G, Young AR, Baron JC. Early postischemic hyperperfusion: pathophysiologic insights from positron emission tomography. J Cereb Blood Flow Metab 1999;19:467–482 [DOI] [PubMed] [Google Scholar]
- 9.Kidwell CS, Saver JL, Mattielo J, et al. Diffusion-perfusion MRI characterization of post-recanalization hyperperfusion in humans et al. Neurology 2001;57:2015–2021 [DOI] [PubMed] [Google Scholar]
- 10.van Dorsten FA, Olah L, Schwindt W, et al. Dynamic changes of ADC, perfusion, and NMR relaxation parameters in transient focal ischemia of rat brain. Magn Reson Med 2002;47:97–104 [DOI] [PubMed] [Google Scholar]
- 11.Macfarlane R, Moskowitz MA, Sakas DE, et al. The role of neuroeffector mechanisms in cerebral hyperperfusion syndromes. J Neurosurg 1991;75:845–855 [DOI] [PubMed] [Google Scholar]
- 12.Chan PH. Role of oxidants in ischemic brain damage. Stroke 1996;27:1124–1129 [DOI] [PubMed] [Google Scholar]
- 13.Fukuyama N, Takizawa S, Ishida H, et al. Peroxynitrite formation in focal cerebral ischemia-reperfusion in rats occurs predominantly in the peri-infarct region. J Cereb Blood Flow Metab 1998;18:123–129 [DOI] [PubMed] [Google Scholar]
- 14.Terada K, Inao K, Mizutani N, et al. Cerebral blood flow, glucose metabolism and tunel-positive cells in the development of ischemia. Cerebrovasc Dis 2001;11:9–19 [DOI] [PubMed] [Google Scholar]
- 15.White BC, Sullivan JM, DeGracia DJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci 2000;179:1–33 [DOI] [PubMed] [Google Scholar]
- 16.Li F, Silva MD, Liu KF, et al. Secondary decline in apparent diffusion coefficient and neurological outcomes after a short period of focal brain ischemia in rats. Ann Neurol 2000;48:236–244 [PubMed] [Google Scholar]
- 17.Li F, Liu KF, Silva MD, et al. Acute postischemic renormalization of the apparent diffusion coefficient of water is not associated with reversal of astrocytic swelling and neuronal shrinkage in rats. AJNR Am J Neuroradiol 2002;23:180–188 [PMC free article] [PubMed] [Google Scholar]
- 18.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke 1981;12:723–725 [DOI] [PubMed] [Google Scholar]
- 19.Memezawa H, Smith ML, Siesjö BK. Penumbral tissues salvaged by reperfusion following middle cerebral artery occlusion in rats. Stroke 1992;23:552–559 [DOI] [PubMed] [Google Scholar]
- 20.Hoehn-Berlage M, Eis M, Back T, Kohno K, Yamashita K. Changes of relaxation times (T1, T2) and apparent diffusion coefficient after the middle cerebral artery occlusion in the rat: temporal evolution, regional extent, and comparison with histology. Magn Reson Med 1995;34:824–834 [DOI] [PubMed] [Google Scholar]
- 21.Strong AJ, Venables GS, Gibson G. The cortical ischemic penumbra associated with occlusion of the middle cerebral artery in the cat, 1: topography of changes in blood flow, potassium ion activity and EEG. J Cereb Blood Flow Metab 1983;3:86–96 [DOI] [PubMed] [Google Scholar]
- 22.Hakim AM, Hogan MJ, Carpenter S. Time course of cerebral blood flow and histological outcome after focal cerebral ischemia in rats. Stroke 1992;23:1138–1144 [DOI] [PubMed] [Google Scholar]