Abstract
Summary: Hibernoma is a rare benign soft tissue tumor composed of remnants of fetal brown fat. It has only rarely been reported in the literature in patients ranging in age from 2 to 75 years. We present a case of multiple hibernomas occurring in a 1-month-old infant.
Hibernoma is a rare benign soft tissue tumor composed of brown fat. We present a case of multiple hibernomas in a 1-month-old female patient and discuss the radiographic, pathologic, and clinical features of this tumor.
Case Report
A 1-month-old term female infant was transferred to our institution because of respiratory distress. Physical examination revealed only “vascular markings” behind the left ear. Laboratory findings were unremarkable. She subsequently underwent flexible bronchoscopy, which demonstrated a significant mass at the subglottic level projecting posteriorly and laterally on the left, resulting in greater than 50% stenosis. No biopsy was performed. A CT scan of the neck was obtained for further evaluation.
Noncontrast neck CT scanning demonstrated a 3-mm ovoid mass within the posterior and left lateral aspects of the subglottic tracheal wall, causing a moderate amount of tracheal narrowing (not shown). It was isoattenuated relative to muscle before contrast medium administration, enhanced homogeneously after contrast medium administration, and was thought to be pathognomonic for a hemangioma.
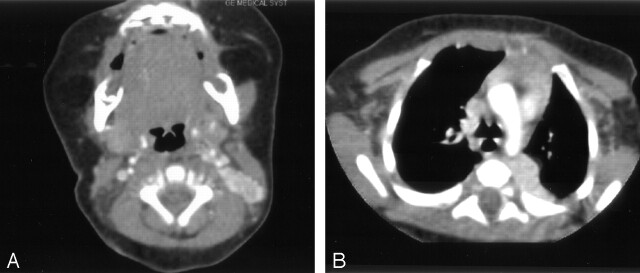
Two other incidental masses were identified on CT scans, however, including a 1.9 × 0.8 cm lateral cervical well-demarcated, oblong soft tissue mass deep to the left sternocleidomastoid muscle. This extended superiorly and medially, interdigitating with the left posterior paraspinal musculature at the C1 level. It was homogeneous and slightly hypoattenuated relative to muscle before contrast material administration (not shown). Following the intravenous administration of iodinated contrast material, avid yet slightly heterogeneous enhancement with multiple small, intensely enhancing peripheral lobulations (Fig 1A) were noted. The third mass was a 1.0 × 2.1 cm left thoracic paraspinal mass with similar imaging characteristics (Fig 1B) to the lateral cervical mass.
Fig 1.
Axial postcontrast CT scans of the neck and chest.
A, Axial postcontrast neck CT scan shows an avidly enhancing, slightly heterogeneous soft tissue mass located deep to the left sternocleidomastoid muscle. This lobulated lesion has an almost “pancreatic-like” appearance. B, Axial postcontrast chest CT scan shows a similar mass in the left paraspinal region, which also enhances avidly with slight heterogeneity.
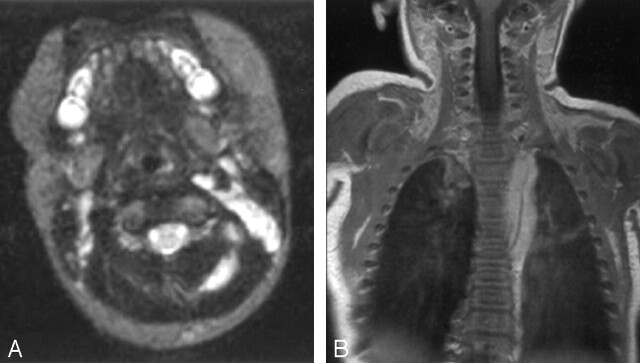
MR examination of the neck demonstrated the lateral cervical mass to have slight T1 and marked T2 prolongation (Fig 2A) and avid, nearly homogeneous enhancement following the intravenous administration of gadolinium (not shown). The large left thoracic paraspinal mass extended from the T2 to the T9–T10 levels and had the same imaging characteristics. In addition, small flow voids were present within this mass (Fig 2B). The subglottic mass was not evaluated by means of MR imaging.
Fig 2.
Axial and coronal MR images of the neck and chest.
A, Axial T2-weighted (TR, 3000; TE, 120) image of the neck shows a nearly uniformly hyperintense mass deep to the left sternocleidomastoid muscle with an extension in between the left posterior paraspinal musculature. The hyperintense area on the contralateral side is a cluster of normal small lymph nodes. B, Postgadolinium coronal T1-weighted (TR, 500; TE, 12) image of the chest shows a diffusely enhancing left paraspinal mass extending in the craniocaudal direction. A prominent flow void within this mass is appreciated on this image and suggests tumor vascularity.
The patient subsequently underwent tracheostomy for respiratory distress. An open surgical biopsy of the left cervical tumor revealed a fleshy, soft mass underlying the transverse process of C7–T1.
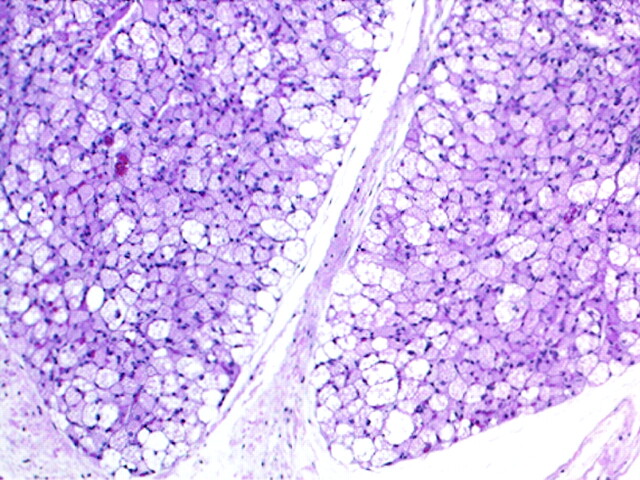
A portion of the mass was submitted for pathologic examination. It grossly comprised lobular tan-brown soft tissue. Microscopic examination revealed well-defined lobules of brown adipose cells with prominent vasculature. These cells were predominantly multivacuolated granular eosinophilic cells with spherical, centrally located nuclei, but more rounded cells with granular eosinophilic cytoplasm were also identified (Fig 3). Mature white adipose cells were absent. The presence of these findings within a mass lesion led to the diagnosis of hibernoma.
Fig 3.
(hematoxylin and eosin, 100× original magnification) The mass comprised lobules of brown adipose cells with prominent vasculature. As seen here, these cells were predominantly multivacuolated granular eosinophilic cells with spherical nuclei.
Discussion
Hibernoma is a rare benign tumor composed of brown fat. It was first described in humans in 1906 by Merkel and named by Gery in 1914. Brown fat occurs normally in the human fetus, being distributed in the neck, axillae, and subpleural regions (1, 2). It may also be present in neonates but will gradually decrease, beginning at about 8 weeks (1). The location of many hibernomas (our case included) corresponds to the fetal distribution. These tumors have been reported to occur most commonly in the interscapular area, neck, axilla, and mediastinum (2), although the thigh was found to be the most common site in a recent review (3). There are also case reports of this tumor in more unusual sites including an intradural extramedullary hibernoma of the cervical spine (4). The peak incidence of hibernoma is in the third or fourth decade of life, but it can be seen at almost any age (2). Reports of sex predilection vary in the literature. Hibernomas are usually painless and characteristically present as firm, mobile, slow-growing masses, sometimes mimicking a lipoma clinically. Hibernoma is not known to metastasize, and to our knowledge, recurrence following complete local excision has not been reported (3).
Although we cannot be certain that the intrathoracic mass is also a hibernoma, because biopsy was not performed, the CT and MR imaging characteristics of these two masses are nearly identical, and we think that it is very likely both are hibernomas. The etiologic factors of our patient’s subglottic mass, on the other hand, are less certain, and the patient is currently undergoing steroid therapy owing to a presumptive diagnosis of subglottic hemangioma.
Grossly, hibernomas are well encapsulated and soft, with a lobular appearance (5). They are typically red or brown. Microscopically, three cell types have been reported, including multivacuolated cells with variably eosinophilic cytoplasm and small central nuclei, smaller granular eosinophilic cells containing very little lipid, and univacuolated adipocytes (6). Abundant mitochondria are seen on electron microscopy (1). Karyotypic aberrations involving chromosome 11 have been implicated in the development of these tumors (1).
Radiographically, CT, MR imaging, sonographic, angiographic, and scintigraphic appearances of this tumor have been described. CT findings are well-defined mass with attenuation values between those of fat and muscle (6), along with a variable amount of enhancement (5). The CT finding of small, intensely enhancing peripheral nodular areas in our case was distinctive and is a potentially useful clue to the diagnosis if it is found to be present in future cases of hibernomas. On MR images, high T1 signal intensity (hyperintense relative to skeletal muscle but slightly hypointense relative to subcutaneous fat) has been the most commonly reported finding (7), although intermediate signal intensity (as in our case) has also been reported (4). On T2-weighted images, high signal intensity similar to that of subcutaneous fat is typical (5), although exceptions have also been reported (2). These tumors do not demonstrate fat suppression (7). Atilla et al (2) have reported minimal chemical shift artifacts with subcutaneous fat, which retrospectively may be present in our case on the axial T1-weighted images of the neck. Following the intravenous administration of gadolinium, hibernomas characteristically demonstrate marked enhancement (4).
The sonographic appearance of hibernoma has been a uniformly hyperechoic mass (5, 7). At angiography, these tumors have rich vascularity and occasional arteriovenous shunting (2, 8). Hibernomas have also been detected incidentally on technetium-99 m tetrofosmin myocardial perfusion studies performed in patients with chest pain, appearing as masslike areas of increased activity (9, 10). Active uptake has also been reported as an F-18 fluorodeoxyglucose positron emission tomography finding (10).
The preoperative differential diagnosis in our case included multiple hemangiomas and neuroblastoma. Biopsy was necessary for diagnosis. The imaging characteristics of this mass did not allow us to consider fat-containing lesions in our differential diagnosis preoperatively. If imaging characteristics point to fat content within such a lesion, a differential diagnosis of atypical lipoma, hibernoma, angiolipoma, or liposarcoma could be entertained (2, 5). The use of chemical-shift (in- and opposed-phase) MR imaging, which has not been described in the literature in association with hibernoma thus far, could perhaps be helpful in demonstrating the lipid component of this mass if it is considered prospectively.
Conclusion
Knowledge of this rare tumor, its distribution, and imaging characteristics may allow one to include it in the differential diagnosis preoperatively. Biopsy is still necessary, however, to confirm a mass as a benign hibernoma.
References
- 1.Chen DY, Wang CM, Chan HL. Hibernoma: case report and literature review. Dermatol Surg 1998;24:393–395 [PubMed] [Google Scholar]
- 2.Atilla S, Eilenberg SS, Brown JJ. Hibernoma: MRI appearance of a rare tumor. Magn Reson Imag 1995;13:335–337 [DOI] [PubMed] [Google Scholar]
- 3.Furlong MA, Fanburg-Smith JC, Miettinen M. The morphologic spectrum of hibernoma: a clinicopathologic study of 170 cases. Am J Surg Pathol 2001;25:809–814 [DOI] [PubMed] [Google Scholar]
- 4.Chitoku S, Kawai S, Watabe Y, et al. Intradural spinal hibernoma: case report. Surg Neurol 1998;49:509–513 [DOI] [PubMed] [Google Scholar]
- 5.Choi J, Heiner J, Agni R, Hafez GR. Case of the season. Semin Roentgenol 2002;37:99–101 [DOI] [PubMed] [Google Scholar]
- 6.Sansom HE, Blunt DM, Moskovic EC. Large retroperitoneal hibernoma: CT findings with pathological correlation. Clin Radiol 1999;54:625–627 [DOI] [PubMed] [Google Scholar]
- 7.Anderson SE, Schwab C, Stauffer E, et al. Hibernoma: imaging characteristics of a rare benign soft tissue tumor. Skeletal Radiol 2001;30:590–595 [DOI] [PubMed] [Google Scholar]
- 8.Balestreri L, Canzonieri V. Case report: axillary hibernoma: radiological and pathological findings of a rare tumour. Clin Radiol 1998;53:853–855 [DOI] [PubMed] [Google Scholar]
- 9.Oller JD, Gomez JD, Kortazar JF, et al. Scapular hibernoma fortuitously discovered on myocardial perfusion imaging through Tc-99m tetrofosmin. Clin Nucl Med 2001;26:69–70 [DOI] [PubMed] [Google Scholar]
- 10.Chatterton BE, Mensforth D, Coventry BJ, Cohen P. Hibernoma: intense uptake seen on Tc-99m tetrofosmin and FDG positron emission tomographic scanning. Clin Nucl Med 2002;27:369–370 [DOI] [PubMed] [Google Scholar]