Abstract
BACKGROUND AND PURPOSE: After the advent of extracellular contrast media, hepatobiliary-specific gadolinium chelates were developed to improve the diagnostic value of MR imaging of the liver. Gadobenate dimeglumine (Gd-BOPTA) is a new paramagnetic contrast agent with partial biliary excretion that produces prolonged enhancement of liver parenchyma on T1-weighted images. However, whether Gd-BOPTA is useful as a contrast agent in central nervous system disease, particularly in brain tumors, is unclear.
METHODS: The behavior of Gd-BOPTA as a brain tumor–selective contrast agent was compared with that of gadopentetate dimeglumine (Gd-DTPA), an MR contrast agent used in central nervous system disease, in a common dose of 0.1 mmol/kg. An MR imaging study of these two contrast agents was performed, and tissue concentrations were measured with inductively coupled plasma atomic emission spectroscopy (ICP-AES).
RESULTS: Gd-BOPTA showed better MR imaging enhancement in brain tumors than did Gd-DTPA at every time course until 2 hours after administration and no enhancement in peritumoral tissue and normal brain. Corresponding results with ICP-AES showed significantly greater uptake of Gd-BOPTA in tumor samples than that in peritumoral tissue and normal brain 5 minutes after administration. Gadolinium was retained for a longer time in brain tumors when Gd-BOPTA rather than Gd-DTPA was administered.
CONCLUSION: Gd-BOPTA is a useful contrast agent for MR imaging in brain tumors and possibly an effective absorption agent for neutron capture therapy.
MR imaging is one of the most useful methods for the diagnosis of brain tumors because of its high resolution and ability to reveal tissue characteristics. After the advent of extracellular contrast media, hepatobiliary-specific gadolinium chelates were developed to improve the diagnostic value of MR imaging of the liver (1). Gadobenate dimeglumine (Gd-BOPTA) is a new paramagnetic contrast agent, with partial biliary excretion, that produces prolonged enhancement of liver parenchyma at T1-weighted imaging (2–4). However, whether Gd-BOPTA is useful as a contrast agent in central nervous system disease, particularly in brain tumors, is unclear.
In this study, we compared the behavior of Gd-BOPTA as a brain tumor–selective contrast agent with that of gadopentetate dimeglumine (Gd-DTPA), an MR contrast agent commonly used in central nervous system disease. We performed an MR imaging study of these two agents as contrast materials, and we measured tissue concentrations with inductively coupled plasma atomic emission spectroscopy (ICP-AES).
The pharmacokinetics of Gd-BOPTA and its distribution in organ tissue have been clearly determined (5). In this study, we report on the efficacy of Gd-BOPTA as a useful contrast agent for MR imaging in brain tumors and possibly as an effective absorption agent for neutron capture therapy (NCT), which is a topic for further research.
Methods
A 0.5-mmol/L solution of Gd-BOPTA (Bracco SpA, Milan, Italy) and a 0.5-mmol/L solution of Gd-DTPA (Magnevist; Schering, Berlin, Germany) were used. The chemical structure of Gd-BOPTA has meglumine as the sole saltifying agent, and a molecular weight of 1058.17. Fisher 344 rats (for MR imaging and ICP study; weight, 200–250 g; n = 6) were used for the experimental brain tumor model. Nine-liter glioma cells (10,000/μL) were implanted into the left caudate nucleus through a small burr hole. A stereotactic manipulator with a 27-gauge fine needle was used for tumor inoculation. Fourteen days after the inoculation of the tumor cells, general anesthesia was induced with pentobarbital (30–50 mg/kg). MR imaging was performed with a 2.4-T superconducting system (BMT 24/40; Bruker Co., Ltd., Karlsruhe, Germany) by using the following parameters: 514/28 (TR/TE); field of view, 4.5 cm; section thickness, 1.6 mm; matrix, 64 × 64; averaging, two times. Serial T1-weighted spin-echo images were obtained before and 5 minutes, 30 minutes, 1 hour, and 2 hours after intravenous injection of Gd-BOPTA and Gd-DTPA at a dose of 0.1 mmol/kg. The time courses of changes in the contrast enhancement of tumors were compared by use of the signal intensity (SI) ratio, which was the average SI of the region of interest in the tumor divided by that of contralateral normal brain. The lesion-to-brain ratio (L/B) was defined as SI brain lesion − SI normal brain. For each animal, the image with the highest L/B contrast enhancement was selected by visual inspection, and a seed point was placed in the tumor region. SI threshold values were adjusted by the operator (T.Z.) until the connected region in the threshold range defined by the program coincided with the enhancing area, as perceived visually. SIs were then recorded for region of interest, which served as a template for the tumor taken for the corresponding animal.
The tumor-bearing rats were sacrificed 5 minutes, 30 minutes, 1 hour, or 2 hours after the injection of Gd-BOPTA and Gd-DTPA at a dose of 0.1 mmol/kg. Tissue specimens, including the tumor peritumoral brain tissue, contralateral normal brain tissue, and blood, liver, spleen, kidney, and muscle were obtained and immediately placed in a deep freezer, where they were kept until used for analysis.
To transfer tissue specimens into liquid in order to measure gadolinium concentration by ICP-AES (IACP757V; Nippon Jarrell-Ash Co. Ltd., Kyoto, Japan), a microwave sample preparation system (MDS-2000; CEM Corp, Matthews, NC) was used. First, we mixed the tissue specimens with 2–3 mL of 10% hydrogen nitrate each. We then put them into a microwave oven (advanced composite vessel) for wet digesting for about 1 hour, after which we obtained the liquid. By using ICP-AES, gadolinium concentration was moved.
Results
MR Imaging Study
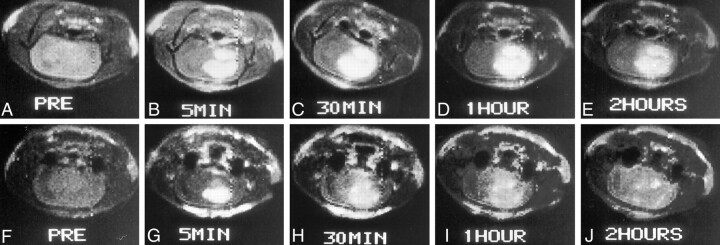
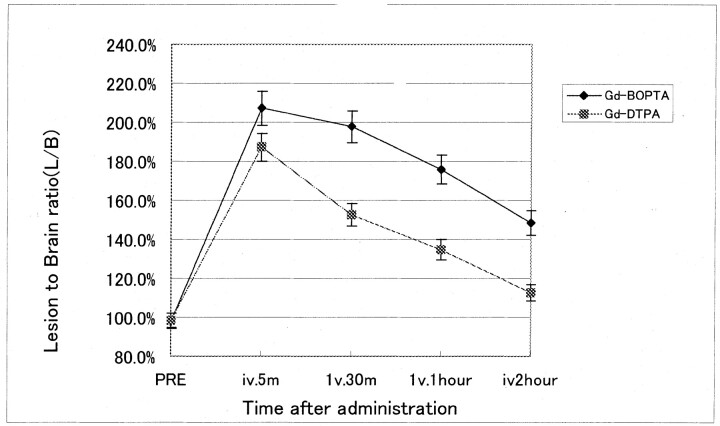
All tumors showed considerable enhancement after use of either contrast agent. Examples of images obtained in this study are shown in Figure 1. At T1-weighted imaging, brain tumor enhancement peaked 5 minutes after administration at 87% ± 6% for Gd-BOPTA and 53% ± 5% for Gd-DTPA. Given that enhancement of normal brain tissue remains negligible (1–3%) after administration of either contrast agent, the stronger lesion enhancement after Gd-BOPTA translated into a higher L/B, which peaked at 2.07 ± 0.09 as compared with 1.87 ± 0.06 after Gd-DTPA administration. Gd-BOPTA retained an L/B of 1.48 ± 0.07 compared with that of Gd-DTPA at 1.13 ± 0.06 2 hours after IV administration (Fig 2). These results reveal that Gd-BOPTA showed better MR imaging enhancement for brain tumor at every time course until 2 hours after administration and no enhancement in peritumoral tissue and normal brain (P < .05).
Fig 1.
Serial T1-weighted spin-echo MR images (514/28) obtained before and at 5 minutes, 30 minutes, 1 hour, and 2 hours after contrast agent administration.
A–E, Gd-BOPTA was the contrast agent used.
F–J, Gd-DTPA was the contrast agent used.
Fig 2.
L/B time profiles after IV administration of 0.1 mmol/kg of Gd-BOPTA and Gd-DTPA during T1-weighted imaging (514/28) at 2.4 T. Data are mean ± SD of six animals.
Inductively Coupled Plasma Study
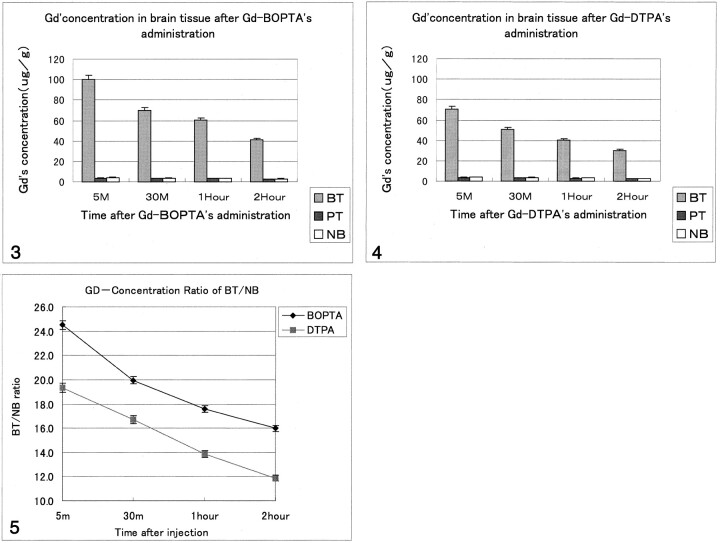
Gadolinium concentration in brain tumor and blood peaked 5 minutes after administration of 0.1 mmol/kg of either Gd-BOPTA or Gd-DTPA, and both decreased continuously from 5 minutes to 2 hours afterward. Both brain tissue specimens had a low gadolinium concentration and showed slowly decreased speed (Figs 3 and 4). However, a higher gadolinium concentration was observed in brain tumor tissue at 5 minutes in the Gd-BOPTA group, and the higher concentration in comparison with the Gd-DTPA group was retained until 2 hours (Table). This could be described as the gadolinium concentration ratio: concentration of gadolinium in brain tumor-concentration of gadolinium in normal brain (BT/NB). The Gd-BOPTA group had a high BT/NB (24.50 ± 1.08) compared with that of the Gd-DTPA group (19.93 ± 1.02) when 0.1 mmol/kg of the agent was administered. Moreover, Gd-BOPTA retained a higher BT/NB 2 hours after administration (16.00 ± 1.07) compared with that for Gd-DTPA (11.87 ± 1.06) (Fig 5). Both agents showed rapid elimination from blood, although Gd-BOPTA had a higher gadolinium concentration. The gadolinium concentration of other organs was highest in the kidney, followed by that in the liver, then the blood, the spleen, brain tumor, muscle, then peritumoral tissue and normal brain. A significantly greater uptake of Gd-BOPTA was observed 5 minutes after administration in tumor samples compared with peritumoral tissue and normal brain tissue, and gadolinium retained longer in brain tumor tissue (until 2 hours with Gd-BOPTA administration), whereas rapid elimination of Gd-DTPA was seen (P < .05).
Fig 3.
Graphic representation of the gadolinium concentration in brain tissue in rats that received Gd-BOPTA. (Gd indicates gadolinium; BT, brain tumor; PT, peritumoral tissue; NB, normal brain.)
Fig 4.
Graphic representation of gadolinium concentration in brain tissue in rats that received Gd-DTPA.
Gadolinium concentration (μg/g) in rat organ tissue after IV 0.1 mmol/mL/kg gadobenate dimeglumine and gadopentetate dimeglumine
| Tissue | Gd-BOPTA |
Gd-DTPA |
||||||
|---|---|---|---|---|---|---|---|---|
| 5 Minutes | 30 Minutes | 1 Hour | 2 Hours | 5 Minutes | 30 Minutes | 1 Hour | 2 Hours | |
| Brain tumor | 100.33 ± 7.91 | 70.21 ± 1.22 | 60.18 ± 2.31 | 40.93 ± 1.83 | 70.39 ± 8.75 | 50.92 ± 6.37 | 40.31 ± 7.42 | 30.22 ± 4.91 |
| Peritumoral tissue | 3.63 ± 0.19 | 3.45 ± 0.29 | 3.40 ± 0.57 | 2.73 ± 0.31 | 3.51 ± 0.22 | 3.30 ± 0.46 | 2.97 ± 0.11 | 2.58 ± 0.27 |
| Normal brain | 4.21 ± 0.69 | 3.61 ± 0.77 | 3.51 ± 0.86 | 3.08 ± 0.82 | 3.82 ± 0.41 | 3.54 ± 0.52 | 3.10 ± 0.42 | 2.71 ± 0.62 |
| Blood | 270.13 ± 29.57 | 81.16 ± 12.31 | 40.49 ± 8.29 | 8.27 ± 0.76 | 229.61 ± 42.73 | 69.02 ± 10.32 | 34.42 ± 6.86 | 7.03 ± 1.87 |
| Liver | 570.12 ± 92.71 | 397.85 ± 49.29 | 149.71 ± 29.62 | 132.46 ± 44.29 | 480.46 ± 78.02 | 327.17 ± 62.34 | 123.37 ± 36.63 | 107.22 ± 46.29 |
| Kidney | 897.28 ± 62.01 | 730.65 ± 88.72 | 590.47 ± 70.91 | 420.16 ± 82.66 | 843.23 ± 97.34 | 793.17 ± 54.71 | 612.23 ± 82.67 | 452.17 ± 93.68 |
| Spleen | 88.38 ± 6.29 | 84.63 ± 9.29 | 60.71 ± 8.65 | 46.32 ± 6.23 | 86.58 ± 3.87 | 80.47 ± 6.28 | 59.23 ± 7.39 | 43.79 ± 5.99 |
| Muscle | 87.15 ± 11.87 | 57.68 ± 10.21 | 14.32 ± 2.76 | 5.37 ± 0.27 | 79.17 ± 2.38 | 48.79 ± 8.66 | 11.73 ± 2.83 | 4.86 ± 0.78 |
Fig 5.
Graphic comparison of BT/NB with Gd-BOPTA versus that of Gd-DTPA.
Discussion
In this study, the stronger enhancement in tumor tissue on T1-weighted images after Gd-BOPTA administration is consistent with a higher relaxation rate of free water due to the higher relaxivity of this chelate. On the basis of our findings (that Gd-BOPTA showed stronger enhancement in brain tumor, had a higher L/B ratio, and retained enhancement longer than did Gd-DTPA), and because of its low toxicity (6, 7), Gd-BOPTA would be a suitable contrast agent for use in brain tumor MR imaging. Normal brain enhancement was negligible in this study. By using ex vivo brain examination, Mascalchi et al (8) also proved no uptake in normal brain. This is also an advantage of obtaining contrast enhancement of tumor relative to normal brain.
In recent years, the therapeutic potential of gadolinium-NCT (Gd-NCT) has been explored in experimental models (9–14). Gd-DTPA, which has been used in Gd-NCT trials, is eliminated rapidly from tumor tissue after intravenous injection; therefore, the neutrons have to be irradiated immediately after intravenous administration (9). The rapid elimination of gadolinium from tumor tissue during irradiation depresses the therapeutic effect. A point for success in current Gd-NCT trials is the use of a device by which gadolinium can be delivered efficiently and retained long enough inside the tumor tissue or cells during thermal neutron irradiation. With Gd-BOPTA administration, gadolinium can be retained for up to 2 hours in brain tumor. Whether Gd-BOPTA enters into the brain cells remains unclear; nevertheless, it might be possible, because Gd-BOPTA enters the plasma membrane of hepatocytes, while Gd-DTPA does not (15). If gadolinium is incorporated into the cells, the radiation effect might be greater than from the extracellular gadolinium compounds. Further research with Gd-BOPTA for a Gd-NCT experiment is planned.
Conclusion
Gd-BOPTA is a useful contrast agent for the MR imaging of brain tumors. It might also be an effective absorption agent for NCT, which is a topic for further research.
References
- 1.Giovagnonia A, Paci E. Liver III: gadolinium based hepatobiliary contrast agents (Gd-EOB-DTPA and Gd-BOPTA/diemeg). Magn Reson Clin North Am 1996;4:61–72 [PubMed] [Google Scholar]
- 2.Gianfranco R, Gianpaolo P, Alberto S. Interim results of phase II clinical testing of gadobenate dimeglumine. Invest Radiol 1994;29(suppl 2):S183–S185 [DOI] [PubMed] [Google Scholar]
- 3.Riccardo M, Giulia M, Richard LB, et al. Delayed MR imaging of hepatocellular carcinoma enhanced by gadobenate dimeglumine (Gd-BOPTA). J Magn Reson Imaging 1999;9:704–710 [DOI] [PubMed] [Google Scholar]
- 4.Kuwatsuru R, Kadoya M, Ohtomo K, et al Clinical late phase III trials of multihance (Gd-BOPTA) for the magnetic resonance imaging of liver tumor in Japan. J Comput Assist Tomogr 1999;23(suppl 1):S65–S74 [DOI] [PubMed] [Google Scholar]
- 5.Spinazzi A, Lorusso V, Pirovano G, et al. Safety, tolerance, biodistribution, and MR imaging enhancement of the liver with gadobenate dimeglumine: results of clinical pharmacologic and pilot imaging studies in nonpatient and patient volunteers. Acad Radiol 1999;6:282–291 [DOI] [PubMed] [Google Scholar]
- 6.Kstdutani N, Sagami F, Tirone P, Morisetti A, Bussi S, Mandella RC. General toxicity study of gadobenate dimeglumine formulation (E7155) (4): 4-week repeated dose intravenous toxicity study followed by 4-week recovery period in dogs. J Toxicol Sci 1999;24(suppl 1):S41–S60 [DOI] [PubMed] [Google Scholar]
- 7.Okuda Y, Sagami F, Tirone P, Morisetti A, Bussi S, Masters RE. Reproductive and developmental toxicity study of gadobenate dimeglumine formulation (E7155)(4): study of embryo-fetal toxicity in rabbits by intravenous administration. J Toxicol Sci 1999;24(suppl 1):S79–S87 [DOI] [PubMed] [Google Scholar]
- 8.Mascalchi M, Jin XN, Agen C, et al. Ex-vivo MR imaging of liver intracellular contrast agents. Magn Reson Imaging 1997;15:469–474 [DOI] [PubMed] [Google Scholar]
- 9.Khokhlov VF, Yashkin PN, Silin DI, Djorova ES, Lawaczeck R. Neutron capture therapy with Gd-DTPA in tumor-bearing rats. In: Mishima Y, ed. Cancer Neutron Capture Therapy. New York, NY: Plenum Press; 1996:865–869
- 10.Matsumura A, Shibata Y, Nakagawa K, et al. Boron-, gadolinium-porphyrin derivatives for neutron capture therapy. In: Mishima Y, ed. Cancer Neutron Capture Therapy. New York, NY: Plenum Press; 1996;245–251
- 11.Hofmann B, Fischer C-O, Lawaczeck R, Platzek J, Semmler W. Gadolinium neutron capture therapy (GdNCT) of melanoma cells and solid tumor with the MRI contrast agent Gadobutrol. Invest Radiol 1999;34:126–133 [DOI] [PubMed] [Google Scholar]
- 12.Akine Y, Tokita N, Tokuuye K, et al. Suppression of rabbit VX-2 subcutaneous tumor growth by gadolinium neutron capture therapy. Jpn J Cancer Res 1993;84:841–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akine Y, Tokita N, Tokuuye K, Satoch M, Kobayashi T, Kanda K. Electron-equivalent dose for the effect of gadolinium neutron capture therapy on the growth of subcutaneously-inoculated Ehrlich tumor cells in mice. Jpn J Clin Oncol 1993;23:145–148 [PubMed] [Google Scholar]
- 14.Akine Y, Tokita N, Matsumoto T, Oyama H, Egawa S, Aizawa O. Radiation effect of gadolinium neutron capture reactions on survival of Chinese hamster cells. Strahlenther Onkol 1990;166:831–833 [PubMed] [Google Scholar]
- 15.Pascolo L, Capelli F, Anelli PL, et al. Molecular mechanisms for hepatic uptake of magnetic resonance imaging contrast agent. Biochem Biophys Res Comm 1999;257:746–7 [DOI] [PubMed] [Google Scholar]