Abstract
BACKGROUND AND PURPOSE: Analysis of specific features in the brain of patients with holoprosencephaly (HPE) may clarify normal and abnormal brain development and help predict outcomes for specific children. We assessed sulcal and gyral patterns of cerebral cortex in patients with HPE and developed a method of grading brain development.
METHODS: Neuroimaging studies (75 MR imaging, 21 CT) of 96 patients with HPE were retrospectively reviewed, with specific attention paid to the cerebral cortex. Thickness of cortex, width of gyri, and depth of sulci were assessed subjectively and by measurement. The angle between lines drawn tangential to the sylvian fissures (“sylvian angle”) was measured in each patient with HPE and in 20 control patients.
RESULTS: Thickness of cortex was normal in all 96 patients. Gyral shape and width and sulcal depth were normal in 80 patients. Twelve patients, all with very severe HPE and microcephaly, had reduced sulcal depth, diffusely in eight and limited to the anteromedial cortex in four with lobar HPE. Four patients had subcortical heterotopia, located anterior to the interhemispheric fissure, associated with shallow sulci in the overlying cortex. Sylvian fissures were displaced further anteriorly and medially as HPE became more severe, until, in the most severe cases, no sylvian fissures could be identified. Sylvian angle measurements corresponded closely with severity of HPE, being largest in the most severe and smallest in the least severe cases. All patients with HPE had sylvian angles significantly larger than the mean of 15° measured in the control patients.
CONCLUSION: The only true malformations of cortical development were subcortical heterotopia. However, diffuse and focal abnormal sulci were observed. We propose our sylvian angle measurement of extent of frontal lobe development as an objective means of quantifying the severity of HPE.
Holoprosencephaly (HPE) is a rare disorder in which induction of the basal forebrain is impaired. Affected children often have severe mental retardation, spastic quadriparesis, athetoid movements, endocrinologic disorders, and epilepsy (1). Some patients are significantly less affected, a fact that led DeMyer and colleagues (1–3) to suggest a classification (from most severe to least severe) of alobar, semilobar, and lobar HPEs. However, some authors have noticed that the precise classification of specific cases of HPE into one of these three categories is not always easy (4, 5). This difficulty has led to an attempt to classify cases of HPE on the basis of specific anatomic features, such as the corpus callosum (6) and the basal ganglia (7). In addition to allowing more detailed correlations with outcomes, this detailed assessment of the anatomic derangements of holoprosencephalic brains has led to insights into the embryologic derangements that lead to HPE; these insights have contributed to our understanding of normal brain development (7, 8). The purpose of our study was to evaluate sulcal and gyral patterns of the cerebral cortex in HPE and develop a quantitative method of grading HPE brain development that could be used in correlating clinical outcome with imaging findings.
Methods
We retrospectively reviewed the imaging studies of 104 patients with HPE who were referred to the Carter Centers for Brain Research in Holoprosencephaly and Related Malformations, a national consortium funded by a nonprofit private foundation, as well as to the authors and their institutions. All patients with the middle interhemispheric variant (9) were excluded from the study, because these patients seem to have a defect in dorsal prosencephalic induction, as compared with the defect in ventral prosencephalic induction in classic HPE (10). The studies included 79 MR imaging and 30 CT studies (five patients underwent both MR imaging and CT). Of these, 75 MR imaging and 21 CT studies were thought by the authors to be adequate for assessing the cerebral cortical gyral pattern, with the result that 96 imaging studies of 96 patients were assessed in the study of the cortex. Seventeen studies in 16 patients (six MR imaging and 11 CT studies, including one patient who underwent both) were considered inadequate for assessing the sylvian fissures (because of motion, thick sections, or poor contrast resolution), so a total of 92 images of 88 patients were the basis of the studies of the sylvian fissures (73 MR imaging and 19 CT studies, with four patients who underwent both). Because these studies were performed at multiple institutions, the precise imaging parameters differed considerably among studies. All MR imaging studies had at least one sagittal sequence and one axial sequence. Coronal sequences were available for 57 patients. CT was performed only in the axial plane, without intravenous administration of contrast material. Only CT scans obtained with a section thickness of ≤5 mm were included. When both CT scans and MR images were available, the MR images, which always had better differentiation of the cortex from the underlying white matter, were used if adequate.
All cases were classified as alobar, semilobar, or lobar HPE. In our experience, the temporal and occipital lobes are the best differentiated in HPE, and the frontal lobes are the least differentiated. Therefore, if no distinct temporal lobes or temporal horns of the lateral ventricles could be identified and no demonstrable interhemispheric fissure was observed, the HPE was classified as alobar. If the temporal lobes were somewhat differentiated, with some true temporal horn formation in the lateral ventricles, or if some true interhemispheric fissure was observed, the HPE was classified as semilobar. If the lateral ventricles were adequately differentiated such that some frontal horn formation was observed, the HPE was classified as lobar. Because most cases (60 of 104 cases) were in the semilobar category and the severity of the brain malformation varied considerably within this category, these cases were further subdivided into severe semilobar and mild semilobar. If a true lateral ventricular body was formed and was observed to be extending superolaterally and anterior to the thalamus, the semilobar HPE was classified as mild. If the only portion of the lateral ventricle that was differentiated was the temporal horn, the semilobar HPE was classified as severe.
The studies were reviewed with respect to the appearance of the cerebral cortex. Specifically, the thickness of the cortex was assessed, both subjectively and by measurement, along with the width of the gyri and the depth of the sulci. Care was taken to note the stage of myelination to avoid misinterpreting the isointensity of the subcortical white matter with the cortex (secondary to incomplete myelination) as abnormally thick cortex; cortex was assessed as thick only if it appeared too thick on both T1- and T2-weighted images. In addition, the location of the sylvian fissures was noted. These fissures were defined as those sulci through which the main branch of the middle cerebral artery (defined as the main lateral branch of the internal carotid artery after its bifurcation) coursed.
The anterior and posterior borders of the sylvian fissures were referred to as the anterior and posterior opercula and were assessed as well defined or poorly defined. A “sylvian line” was defined bilaterally by two points: the inner cortical margin of the most anterior and posterior portions of the insula, defined as the cortex lining the medial aspect of the sylvian fissure, as defined above (Fig 1), essentially a tangent along the insula. The angle formed by the anterior intersection of the sylvian lines, called the sylvian angle, was measured to the closest 5th degree. To determine the normal sylvian angle, it was measured on MR images of 20 control patients between birth and 10 years of age who were studied for a variety of causes but had no atrophy, hydrocephalus, or mass. These included eight children with neurofibromatosis type 1 but no parenchymal abnormalities, seven infants who underwent imaging for a study of normal development, and five children with congenital melanotic nevi in whom no intracranial abnormality was discovered.
Fig 1.
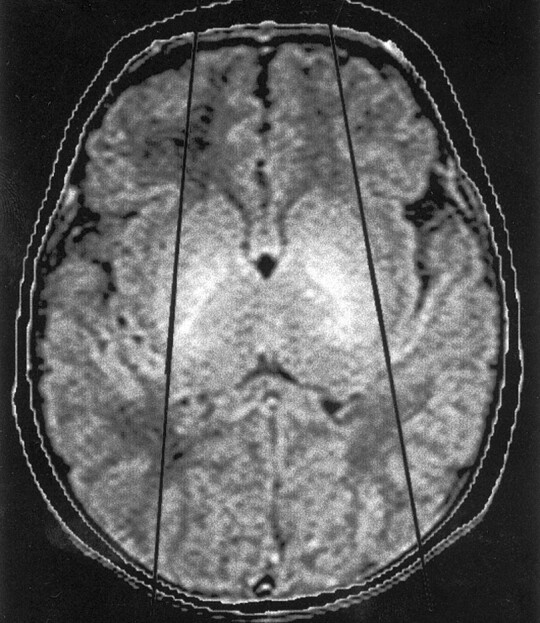
Axial spin-echo (3000/120 [TR/TE]) MR image shows the sylvian angle in a healthy neonate. Black lines are drawn tangential to the sylvian fissures at the level of the basal ganglia. The anterior angle is the sylvian angle.
The two-sample t test assuming unequal variances was used to test whether the sylvian angle was associated with severity of HPE as assessed by the DeMyer classification. We also simplified the groups by combining those with mild semilobar HPE and those with lobar HPE into a “less dysplastic” group and by combining the patients with severe semilobar HPE and those with alobar HPE into a “more dysplastic” group. The two-sample t test assuming unequal variances was then used to determine whether the sylvian angles in these two groups were significantly different. The values obtained in the HPE cases were also compared with the defined normal range in our control population.
Results
Normal Sylvian Angle
The sylvian angles of the control patients ranged from 12° to 18° (mean 15° ± 1.1° [SD]).
Sylvian Fissures in Association with Holoprosencephaly
Sylvian fissures were identified in 76 of the 96 patients. Of the 20 patients in whom no sylvian fissures were identified, 16 had alobar HPE and four had semilobar HPE. Two of the cases of semilobar HPE were classified as more dysplastic and two as less dysplastic. The two classified as less dysplastic had significant basal ganglia noncleavage, another marker of severe HPE (7), suggesting that our criteria for degree of severity may have been flawed in these cases. When the sylvian fissures were absent, the internal carotid arteries coursed normally through the cavernous sinuses, then branched into multiple small vessels as they entered the subarachnoid space, without definable anterior cerebral and middle cerebral artery trunks. These small vessels seemed fairly evenly distributed over the cerebrum.
The mean sylvian angle for the nine alobar HPE cases in which sylvian fissures were identified was 122° ± 50°. For the 43 semilobar HPE cases in which sylvian fissures were identified, the mean sylvian angle was 77° ± 34°: 101° ± 26° for the 16 severe semilobar cases and 63° ± 30° for the 27 mild semilobar cases. For the 16 patients with lobar HPE, the mean sylvian angle was 39° ± 19°. The mean sylvian angles for the more (25 patients) and less (43 patients) dysplastic groups were 109° ± 37° and 54° ± 29°, respectively. (See comments in the Statistical Analyses section.)
Subjective volume of cerebrum anterior to the sylvian fissures diminished as the sylvian angles became larger (Figs 2–4), as if progressively less frontal lobe were present in these patients. As a consequence, the sylvian fissures seemed to be positioned progressively more anteriorly within the cerebrum. As the sylvian angle approached 180°, no anterior opercula were identified and no gyri were observed between the fissures, as if they were merging. At this time, the merged sylvian fissures occupied the most anterior location of the cerebrum. A single, anterior, midline sylvian fissure was observed in each of two patients, bordered only by bilateral posterior opercula (Fig 5).
Fig 2.
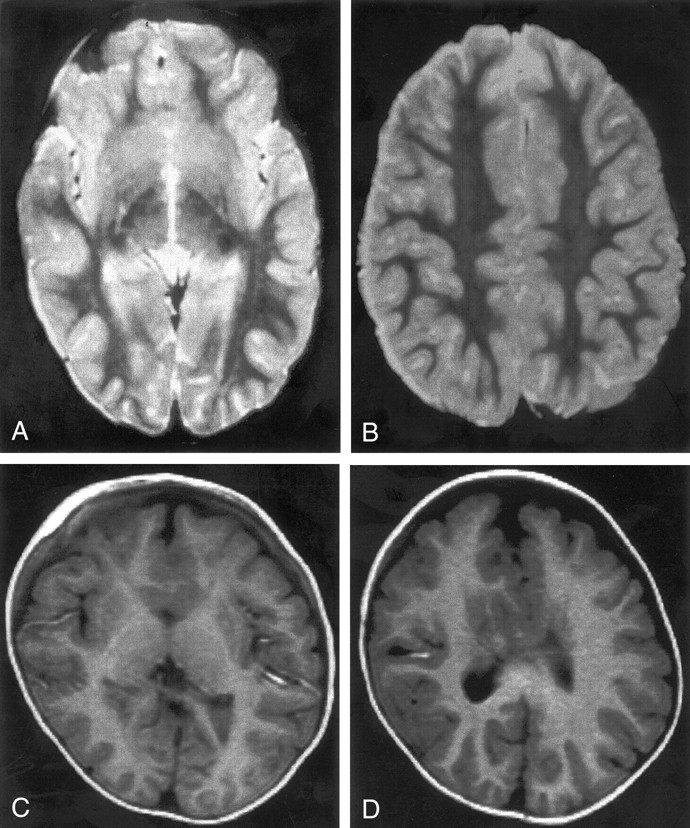
MR images show two cases of mild HPE. Both cases were classified as lobar HPE and have sylvian angles of 25°. Both have small, dysplastic frontal horns and both have a large amount of brain tissue anterior to the sylvian fissures.
A and B, Spin-echo (2500/120) MR images show normal-appearing sulci and gyri in the posterior two-thirds.
C and D, Inversion-recovery (2000/800/inversion time, 12 ms) MR images show normal-appearing sulci and gyri in the posterior half. The medial frontal sulci appear dysplastic.
Fig 4.
A and B, Spin-echo (3000/120) MR images show HPE of moderate severity, classified as less dysplastic semilobar HPE. The sylvian angle is 110°. Only two gyri (solid white arrows) are positioned between the sylvian fissures anteriorly. The basal ganglia are less well developed than those shown in Figure 3, being only a crescent of gray matter anterior to the thalami in B. A thin curvilinear area of gray matter hypointensity (small black arrows, B) is seen between the basal ganglia and presumed insula; this is thought to represent claustrum. Two foci of hypointensity (open white arrows, A) are seen posterior to the basal ganglia crescent but anterior to the thalami in A. These are thought to represent myelinated white matter in the internal capsules.
Fig 5.
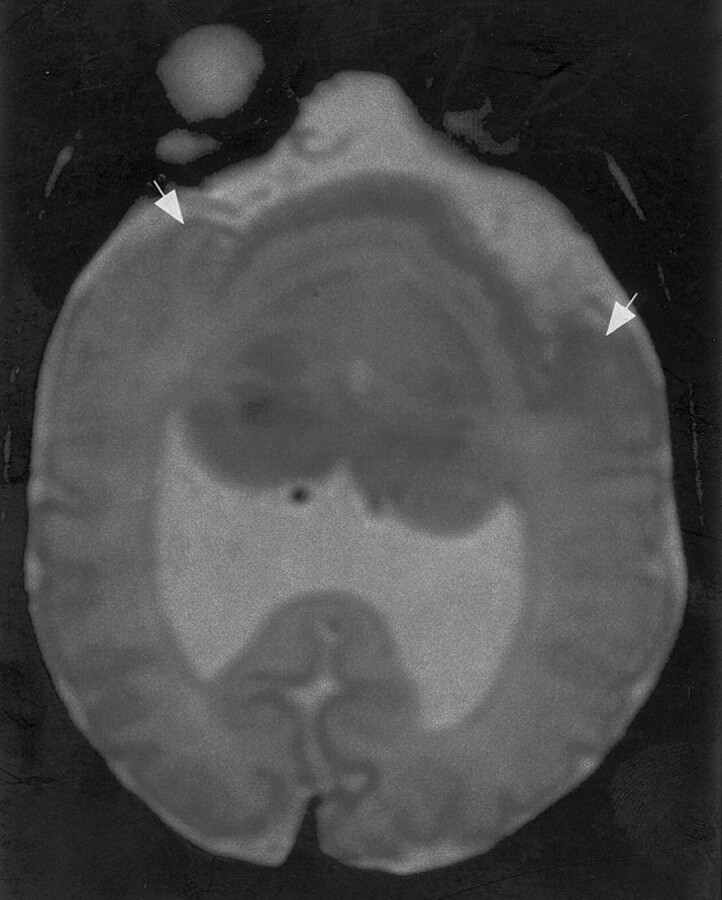
Fast spin-echo (3600/95) MR image shows HPE of moderate severity, classified as more dysplastic semilobar HPE. The anteriormost portion of the cerebrum appears to be a single continuous sylvian fissure. This fissure appears to be composed of the posterior halves of two sylvian fissures, with bilateral posterior opercula (arrows) forming the lateral borders, as if the anterior halves of the fissures had never formed and the posterior halves merged together.
Appearance of the Cortex: Normal Gyral Thickness and Sulcal Depth
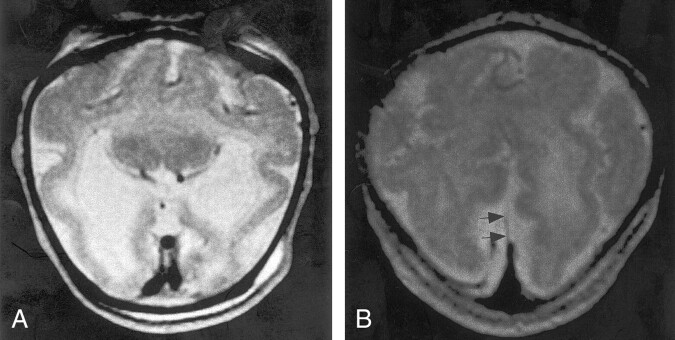
In 80 of the 96 cases of HPE in which the appearance of the cortex could be adequately assessed, the cortical thickness and the width of the gyri were normal. The sulci were of nearly uniform depth in patients with more severe HPE, whereas those with less severe HPE had more normal variation in sulcal depth. Some of the cases of milder semilobar and lobar HPE had a fairly normal gyral pattern in the posterior cerebrum, and some characteristic sulci, such as the calcarine, cingulate, and central sulci, were identified (Fig 2). In the more severe semilobar and alobar cases, no normal gyri were recognized (Fig 6).
Fig 6.
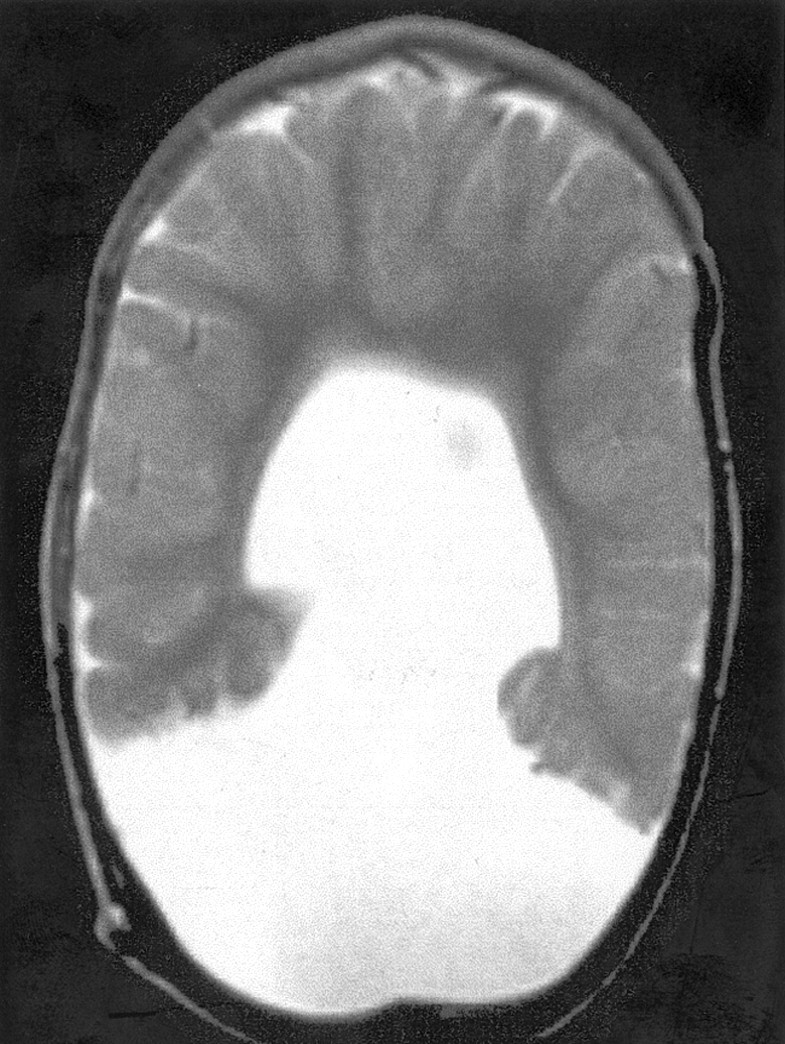
Spin-echo (2500/70) MR image shows severe HPE. No sylvian fissures are identified and, thus, no sylvian angle measured. Gyri have normal, uniform width, and sulci have normal, uniform depth. No specific gyri or sulci are identified.
As the sylvian angle increased, small gyri in the inferior midline were observed in the most anterior aspect of the cerebrum. Analysis showed that these gyri formed the anterior walls of the sylvian fissures (Fig 4); they were thus considered to represent anterior opercula. In most cases, these gyri were small, few in number, and symmetric with respect to the midline. However, in each of four patients, the cerebral tissue anterior to the sylvian fissures was composed of a narrow stalk that then widened into a mushroom-shaped mass of brain tissue that had normal cortical thickness and shallow sulci similar to the appearance of the remainder of the cerebrum (Fig 7).
Fig 7.
MR images show HPE of moderate severity, classified as less dysplastic semilobar. The sylvian angle is 55°.
A, Axial fast spin-echo (4000/84) MR image shows that a mushroom-shaped region of brain (arrowheads) grows anteriorly from the region between the anterior aspects of the sylvian fissures.
B, Coronal spoiled gradient-recalled acquisition in a steady state (36/13) MR images show the large mushroom-shaped region (arrows, B) lying below the remainder of the cerebrum in the most anterior aspect of the calvaria.
Appearance of the Cortex: Abnormal Gyri and Shallow Sulci
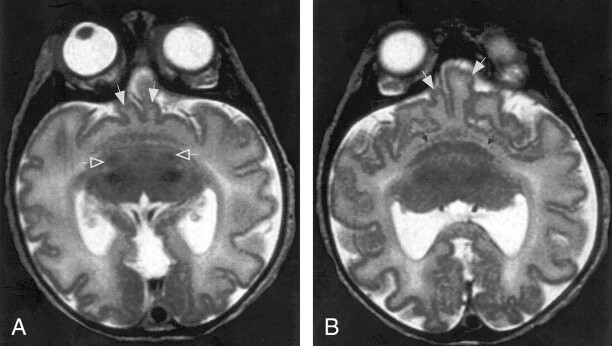
In 12 cases, the gyral pattern was frankly abnormal. In all 12, the sulci were shallow and the gyri were abnormal. In eight cases, the cortex was diffusely abnormal, whereas in four, the abnormality was limited to the midline frontal cortex. The eight cases with diffusely abnormal cortices all had broad gyri with too few sulci. Although the cortex appeared too thick on initial observation, measurements showed a cortical thickness of approximately 3 mm, which is normal (11). All eight cases were classified as alobar HPE; all included large dorsal cysts, little separation of deep gray nuclei, and very little cerebral white matter (Fig 8). The four cases with localized dysplastic cortices were classified as lobar HPE. In each of these cases, the medial frontal cortex was thickened and the gyral pattern was bizarre with tortuous, convoluted sulci, giving an odd appearance on the axial and sagittal images (Fig 2).
Fig 8.
MR images show severe HPE, classified as alobar, with sylvian angle of 135°. First impression of the cerebral cortex is one of pachygyria, but the cortical thickness measures only 3 mm. The cortex appears thick because of the profound microcephaly. This case illustrates some of the difficulties of the DeMyer classification, as the interhemispheric fissure and ventricular systems are much better developed than the basal ganglia and white matter. The basal ganglia, might be considered alobar HPE, but the interhemispheric development suggests mild semilobar HPE.
A, Axial fast spin-echo (4000/90) MR image shows apparently thickened cortex with few gyri and shallow sulci. Thalami are incompletely separated, and no basal ganglia are seen, nor were they seen at other levels.
B, Axial fast spin-echo (4000/90) MR image obtained at a higher level shows an interhemispheric fissure spanning the posterior two-thirds of the cerebrum; the falx cerebri (arrows) is present posteriorly.
Other Cortical Malformations
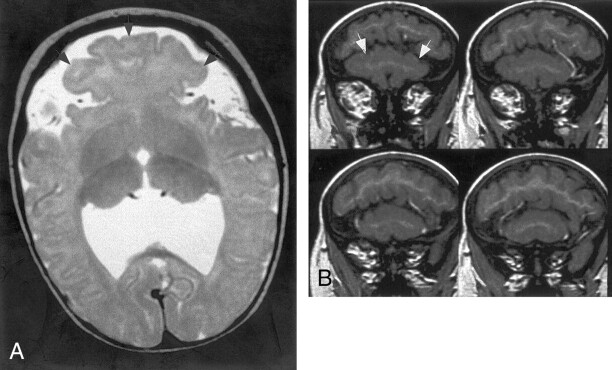
Four patients had subcortical heterotopia that were large, crossed the midline, and were located anterior to the anterior termination of the interhemispheric fissure. All of the heterotopia were connected by a “bridge” of gray matter to the cortex lining the anteriormost interhemispheric fissure and to the frontal cortex in at least one location (Fig 9). The overlying cortex had very shallow sulci.
Fig 9.
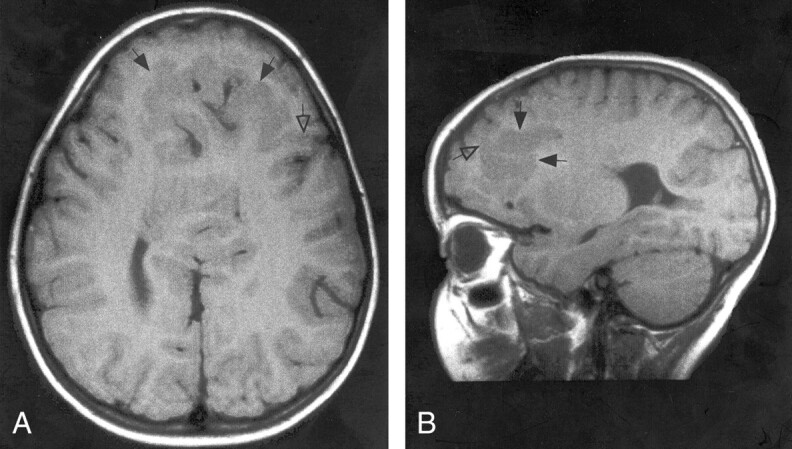
A and B, Spin-echo (700/16) MR images show mild HPE, classified as lobar, with a sylvian angle of 25°. Axial (A) and sagittal (B) views show curvilinear subcortical heterotopia (solid arrows) in the anterior cerebrum. The heterotopia connect with the overlying cortex in several places (open arrow). The overlying cortex is thin with shallow sulci.
Statistical Analyses
The sylvian angles in the alobar and semilobar cases (P = .03), of the lobar and semilobar cases (P < .001), of the mild and severe semilobar cases (P < .001), and of the more dysplastic and less dysplastic cases (P < .001) of HPE were all significantly different, using the two-sample t test, assuming unequal variances. All HPE groups differed significantly from the control group (P = .0001 for the lobar HPE group, which was closest to normal).
Discussion
To our knowledge, this is the first study of the cortical gyral patterns associated with HPE in a large population studied with modern neuroimaging methods. We found that most patients with HPE have normal cortical thickness without definite abnormalities in the depth of sulci or width of gyri. Not surprisingly, the sulcal pattern of the brain was more normal in the less severely affected brains: lobar HPE was more normal than was semilobar HPE, and semilobar HPE was more normal than was lobar HPE. Also, considering that the severity of malformation has an anterior-to-posterior gradient in HPE, with the anterior portions being the least well formed (7, 8, 12), it is not surprising that the gyral pattern of the posterior cerebrum more closely resembled the normal gyral pattern than that of the anterior cerebrum. In less severe HPE, the gyral pattern of the occipital and posterior parietal lobes was normal or nearly normal.
We did not attempt to precisely name the gyri present in our cases of HPE. Our analysis of nearly 100 cases of HPE showed us that assigning the precise names of normal gyri and sulci is difficult in cases of HPE, because major portions of the brain are missing and the portions that are present are usually in far different locations and have different configurations than their counterparts in normal brain. It is often difficult to be certain which lobes are present and which are absent because of the severe distortion of the anatomy. This problem is best illustrated in the mushroom-shaped gyri observed in four patients and illustrated in Figure 7. We were not able to assign names of any normal gyri in such areas.
In this study, we primarily focused on the sylvian fissures in an attempt to show the relationship of the gyral pattern in holoprosencephalic brains to that in normal brains. A surprising, and perhaps useful, result of our analysis was our finding that the relative location and orientation of the sylvian fissures varied with the severity of the HPE. In the least severe cases of HPE, the sylvian fissures were located near the middle of the anteroposterior axis of the brain and the sylvian angle was only slightly increased (Fig 2). As the severity of the HPE increased, the sylvian fissures were found more and more anteriorly with respect to the front of the brain and the amount of cerebral tissue between them diminished (Figs 3–5). As a result of this diminution of tissue, the angle between the fissures increased from 15° in control patients to a mean of 39° in patients with lobar HPE, to 63° in patients with mild semilobar HPE, to 101° in patients with severe semilobar HPE, to 122° in patients with alobar HPE. That this increasing sylvian angle results from a steady decrease in the quantity of frontal lobe anterior to the sylvian fissures is supported by the cases in which a single sylvian fissure was observed in the anterior midline. In these brains, this fissure seems to be composed of the posterior halves of two sylvian fissures, with bilateral posterior opercula forming the lateral borders (Fig 5), as if the anterior halves of the fissures had never formed and the posterior halves merged together. In the most severe cases of HPE, no sylvian fissures could be identified at all. We postulate that in these patients, only those parts of the brain that are normally located posterior or inferior to the sylvian fissures were induced to develop.
Fig 3.
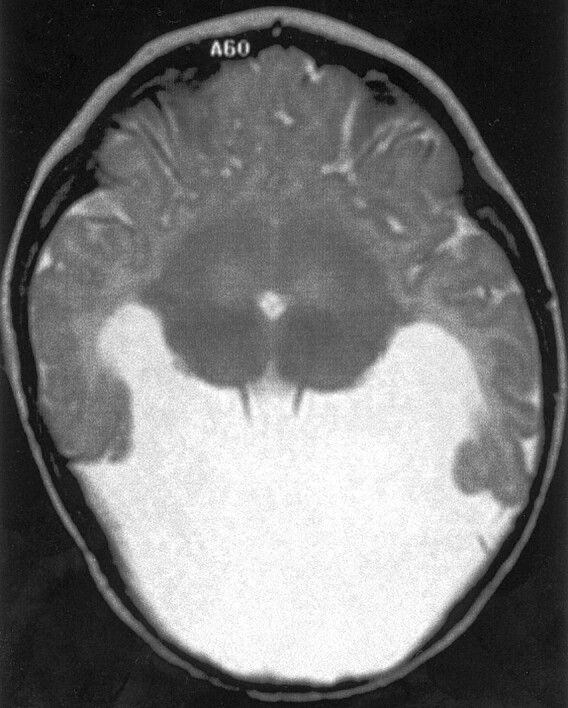
Spin-echo (3000/120) MR image shows HPE of moderate severity, classified as less dysplastic semilobar HPE. The sylvian angle is 95°, and little brain tissue lies anterior to the sylvian fissures.
The sylvian angle may provide a simple objective criterion for classifying cases of HPE: the greater the sylvian angle is, the more severe the HPE. In measuring HPE severity by sylvian angles, patients with no identifiable sylvian fissures would be considered the most severely affected, possibly referred to as having “asylvian” HPE. Beyond this group, there would be no need for specific terms such as lobar or alobar, severe or mild. The case could be classified on the basis of the sylvian angle itself. For some of our patients, assessment by sylvian angle was very useful, because they had severe brain abnormalities despite interhemispheric fissures that extended to the anterior third of their cerebrum and lateral ventricles that had obvious temporal horns and bodies.
An example is illustrated in Figure 8. Using the DeMyer classification and looking at the extent of interhemispheric fissure and of the ventricular system, one would have classified cases such as this as mild semilobar HPE. However, assessment of the cortex and deep gray nuclei of these brains suggests that they are, in fact, very severe cases; the severity is confirmed by the sylvian angle of 135°. Another problem with the DeMyer classification is that the HPEs are a continuum of abnormality, yet the classification forces one to put each brain in a specific category. Two extremely similar brains may be classified differently, for example, one as semilobar and the other as lobar, on the basis of a slight difference in frontal horn formation, despite otherwise being very similar. In contrast, measuring their sylvian angles of 45° and 50° (the HPE with a sylvian angle of 50° is more severe than that of 45°) will show the difference while emphasizing the similarity. Obviously, before the sylvian angle is used for a criterion of severity of HPE, it must first be correlated with developmental milestones to determine whether an association exists. This correlation is currently beginning in our group.
The concept of lack of formation of the anterior frontal lobes, implied by our sylvian angle results, is supported by the cytoarchitectonic analysis presented by Yakovlev (13) in an alobar holoprosencephalic brain. He reported that the typical motor cortex is found in the cortex of the anterior midline. Adjacent to the motor cortex on both sides is a cytoarchitectonic pattern, typical of parietal cortex. Next is the limbic cortex, which borders the crescentic holoventricle. This cytoarchitectonic analysis can be interpreted to indicate that the large area of frontal cortex that normally lies anterior to the motor strip is never induced to form in alobar HPE. Such lack of induction is consistent with the current theory of the embryogenesis of HPE, which postulates a defect in dorsoventral patterning at the most rostral end of the neural tube (5, 7, 8). This theory has been adequately expounded in the recent literature and need not be repeated here. Certain findings in the analyses of our patients also support this concept of the motor cortex being in the anteriormost cerebral cortex in severe cases of HPE. In many of our severe cases of semilobar HPE, we saw myelinated white matter tracts coursing toward the most anterior cortex (Figs 4 and 5). Considering that the only myelinated cerebral white matter tracts in the neonate are the corticospinal tracts (14, 15), it seems highly likely that these myelinated tracts are coursing toward the precentral and postcentral gyri. In addition, in many of our patients, we saw thin strips of gray matter between the basal ganglia and the cortex (presumed insula) lining the presumed sylvian fissure; we suspect that these structures represent the claustra. As the claustrum lies between the corticospinal tract and the insula in the normal brain, the presence of a claustrum-like structure immediately beneath what we have called the sylvian fissure provides additional evidence that we have correctly identified the sylvian fissure and that the motor strip lies deep to that fissure.
If the sylvian fissures are indeed markers of the motor cortex, then these might be used as markers to help identify eloquent areas of cortex in cases of HPE. This could be proved if functional studies such as blood oxygen level-dependent imaging or magnetic source imaging could be performed in patients with HPE. Although the performance of studies that require cooperation and active participation are extremely difficult in most patients with HPE, this may be possible in some of the less severely affected patients.
We also attempted to identify and characterize cortical abnormalities in our patients. Twelve (12%) of 96 patients were considered to have abnormal gyral patterns. Of these 12 patients, eight had diffusely shallow sulci with broad gyri (Fig 8), a pattern that qualifies, by definition, as pachygyria (16). Interestingly, all eight patients had severe HPE and were significantly microcephalic (head circumference more than 2 SDs below normal); no sylvian fissures could be identified in three, and the others all had sylvian angles of ≥110°. Seven cases were classified as alobar HPE using the DeMyer classification; the eighth was classified as semilobar HPE but had a sylvian angle of 110° and essentially no separation of deep gray nuclei. Both of these findings and the severe microcephaly suggest very severe HPE, another illustration of the limitations of the DeMyer classification. It is possible that the abnormal sulcation in these patients results from impaired neuronal migration, possibly as a result of the severe overall disturbance of brain development. Advances in modern molecular biology are making it clear that many factors are involved in proper cortical development (17), and it would not be surprising if some of these were affected in HPE. However, the finding of normal cortical thickness makes abnormal migration seem unlikely. Another possibility is the development of too few white matter tracts in these patients as a result of the profoundly disturbed brain malformation. As sulci are postulated to form as a result of tension on the developing cortex from the afferent and efferent axons (18), a paucity of white matter tracts could result in sulci that are too few and too shallow. It is hoped that the analysis of animal models will clarify some of the mechanisms underlying this diffuse abnormality of sulcation.
In the remaining cases that included cortical abnormalities (in the absence of subcortical heterotopia), those abnormalities were restricted to the medial frontal cortex. These cases had sylvian angles ranging from 25° to 50° (mean, 36°), and all were classified as lobar HPE based on the DeMyer classification; they were among the least severely involved of our 96 cases. We think that the abnormal gyri in these cases were the result of the known back-to-front gradient of developmental disturbance in classic HPE. This gradient was shown in our cases of lobar HPE as better differentiated sulci and gyri posteriorly and less differentiated sulci and gyri anteriorly. The cortical anomalies were identified in those cortical areas that are least well differentiated. We suspect that these cortical anomalies were the result of the incomplete induction of the structures of the most rostral telencephalon. Specifically, lack of formation of rostral axonal pathways may impair sulcation, as described here. Alternatively, lack of formation of the interhemispheric mesenchyme could impair cortical development because of the absence of normal mesenchyme-ectoderm interactions (19).
One might ask why abnormal sulci were observed only in the most severe and least severe cases of HPE; why not in those cases of intermediate severity? Attempts at explaining why the most and least severe cases have abnormal gyri have already been rendered. It is important to remember that the brains of patients with HPE have their greatest abnormalities in the anterior midline. Cases of intermediate severity have no cortex in the anterior midline, because the interhemispheric fissure is not formed anteriorly. Therefore, no abnormal cortex is observed in these patients. It is only in the least severe cases that an interhemispheric fissure is present anteriorly; with the interhemispheric fissure comes anteromedial cortex. This is the cortex that was identified as abnormal in our patients.
Subcortical heterotopia was the other malformation of cortical development that we detected, observed in four (4%) of the 96 patients. These heterotopia were similar to those described in the non-HPE population (20, 21), composed of curvilinear, slightly irregular masses of gray matter that contact the cerebral cortex in at least one location. We did not identify any regions of polymicrogyria, subependymal heterotopia, band heterotopia, or other malformations of cortical development in our patients. The mechanism by which subcortical heterotopia develop is unknown.
Conclusion
An analysis of the cerebral cortex in patients with classic HPE reveals that most patients with HPE have cortices of normal thickness with normal-sized gyri and sulci. Patients with severe HPE and microcephaly may have shallow sulci that are reduced in number; although the cortex appears thick in these patients, measurements show it to be normal. Patients with mild HPE may have abnormally shaped gyri in the medial frontal cortex. The only malformation of cortical development identified was subcortical heterotopia. Finally, the sylvian fissures seem to be displaced further and further anteriorly and medially as HPE becomes more severe, to the point at which, in the most severe cases, no sylvian fissures can be identified. We have defined a measurement, which we call the sylvian angle; we propose this measurement as an objective means of quantifying the severity of HPE.
Footnotes
Funded in part by The Carter Centers for Brain Research in Holoprosencephaly and Related Malformations.
References
- 1.DeMyer W. Holoprosencephaly (cyclopia-arhinencephaly). In: Myrianthopoulos N, ed. Malformations. New York: Elsevier;1987. :225–244
- 2.DeMyer W, Zeman W. Alobar holoprosencephaly (arhinencephaly) with median cleft lip and palate: clinical, nosologic, and electroencephalographic considerations. Confin Neurol 1963;23:1–36 [DOI] [PubMed] [Google Scholar]
- 3.DeMyer W, Zeman W, Palmer CG. The face predicts the brain: diagnostic significance of median facial anomalies for holoprosencephaly (arhinencephaly). Pediatrics 1964;34:256–263 [PubMed] [Google Scholar]
- 4.Probst FP. The Prosencephalies: Morphology, Neuroradiological Appearances and Differential Diagnosis. Berlin: Springer-Verlag; 1979:46
- 5.Golden JA. Towards a greater understanding of the pathogenesis of holoprosencephaly. Brain Dev 1999;21:513–521 [DOI] [PubMed] [Google Scholar]
- 6.Oba H, Barkovich AJ. Holoprosencephaly: an analysis of callosal formation and its relation to development of the interhemispheric fissure. AJNR Am J Neuroradiol 1995;16:453–460 [PMC free article] [PubMed] [Google Scholar]
- 7.Simon E, Hevner R, Pinter J, Kinsman S, Hahn J, Barkovich AJ. Assessment of the deep gray nuclei in holoprosencephaly. AJNR Am J Neuroradiol 2000;21:1955–1961 [PMC free article] [PubMed] [Google Scholar]
- 8.Golden JA. Holoprosencephaly: a defect in brain patterning. J Neuropathol Exp Neurol 1998;57:991–999 [DOI] [PubMed] [Google Scholar]
- 9.Barkovich AJ, Quint D. Middle interhemispheric fusion: an unusual variant of holoprosencephaly. AJNR Am J Neuroradiol 1993;14:431–440 [PMC free article] [PubMed] [Google Scholar]
- 10.Simon EM, Hevner R, Pinter JD, et al. The middle interhemispheric variant of holoprosencephaly. AJNR Am J Neuroradiol (in press) [PMC free article] [PubMed]
- 11.Barkovich AJ, Koch TK, Carrol CL. The spectrum of lissencephaly: report of ten cases analyzed by magnetic resonance imaging. Ann Neurol 1991;30:139–146 [DOI] [PubMed] [Google Scholar]
- 12.Cohen MM Jr, Jirasek JE, Guzman RT, Gorlin RJ, Peterson MQ. Holoprosencephaly and facial dysmorphia: nosology, etiology, and pathogenesis. Birth Defects Orig Artic Ser 1971;7:125–135 [PubMed] [Google Scholar]
- 13.Yakovlev PI. Pathoarchitectonic studies of cerebral malformations, III: arrhinencephalies (holotelencephalies). J Neuropathol Exp Neurol 1959;18:22–55 [DOI] [PubMed] [Google Scholar]
- 14.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, ed. Regional Development of the Brain in Early Life. Oxford: Blackwell;1967. :3–70
- 15.Flechsig P. Anatomie des menschlichen Gehirns und Rückenmarks auf myelogenetischer Grundlage. Leipzig: Georg Thieme; 1920
- 16.Norman MG, McGillivray BC, Kalousek DK, Hill A, Poskitt KJ. Congenital Malformations of the Brain: Pathologic, Embryologic, Clinical, Radiologic and Genetic Aspects. Oxford: Oxford University Press;1995. :223–307
- 17.Gleeson JG, Walsh CA. Neuronal migration disorders: from genetic diseases to developmental mechanisms. Trends Neurosci 2000;23:352–359 [DOI] [PubMed] [Google Scholar]
- 18.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 1997;385:313–318 [DOI] [PubMed] [Google Scholar]
- 19.von Knebel Doeberitz C, Sievers J, Sadler M, et al. Destruction of meningeal cells over the newborn hamster cerebellum with 6-hydroxydopamine prevents foliation and lamination in the rostral cerebellum. Neuroscience 1986;17:409–426 [DOI] [PubMed] [Google Scholar]
- 20.Barkovich AJ. Subcortical heterotopia: a distinct clinicoradiologic entity. AJNR Am J Neuroradiol 1996;17:1315–1322 [PMC free article] [PubMed] [Google Scholar]
- 21.Barkovich AJ. Morphologic characteristics of subcortical heterotopia: MR imaging study. AJNR Am J Neuroradiol 2000;21:290–295 [PMC free article] [PubMed] [Google Scholar]