Abstract
BACKGROUND AND PURPOSE: Proximal occlusion of the parent artery has been widely used for treatment of vertebral dissecting ruptured aneurysms, but this does not always completely prevent rerupture. We retrospectively studied 24 consecutive patients for clinical characteristics and/or for efficacy of occlusion with detachable coils at the site of dissection.
METHODS: During a 45-month period, 24 of 242 patients with aneurysms associated with subarachnoid hemorrhage had dissecting vertebral aneurysms identified at angiography. Eighteen of the 24 patients were treated with platinum coil occlusion at the affected site as early as possible after diagnosis, two patients were treated conservatively, and four others were not eligible for treatment owing to intractable elevation of intracerebral pressure and severe brain stem dysfunction.
RESULTS: The rate of aneurysmal rupture in the posterior fossa was high, at 56 (23%) of the 242 aneurysms, including 24 (10%) vertebral dissecting aneurysms. Subsequent rupture occurred in 14 (58%) of the patients, all within 24 hours after the first attack and three during transportation to the hospital. In all 18 patients, coil embolization at the affected site was successful, with no complications. Radiologic findings showed complete occlusion of the dissection site and patency of the unaffected artery (mean follow-up, 9 months). Among the six patients who did not undergo embolization, only one survived with a good outcome, the others died of repeat hemorrhage.
CONCLUSION: A high rate of vertebral artery dissecting aneurysms may be expected in patients with subarachnoid hemorrhage, especially in those with early repeat hemorrhage. Detachable platinum coil embolization may be more effective than proximal occlusion for treatment of ruptured vertebral dissecting aneurysms because of immediate cessation of blood flow to the dissection site; however, in patients with bilateral dissections or hypoplastic contralateral vertebral arteries, prior bypass surgery or stent placement to preserve the artery will be needed.
The number of dissecting vertebral artery aneurysms occurring with subarachnoid hemorrhage is unknown (1), and evaluation of clinical characteristics in large series has been limited (1, 2). We therefore undertook a retrospective study of vertebral artery dissections occurring in a consecutive population of patients with ruptured aneurysms at our institution.
Treatment of ruptured dissecting vertebral artery aneurysms with proximal occlusion of the parent artery by means of endovascular techniques or clipping has been widely used, but these procedures are not always completely effective in preventing rerupture (3–8). In this context, we assessed the comparatively long-term efficacy of endovascular coil embolization at the dissection site in a consecutive series of patients treated at our institution.
Methods
Patient Population
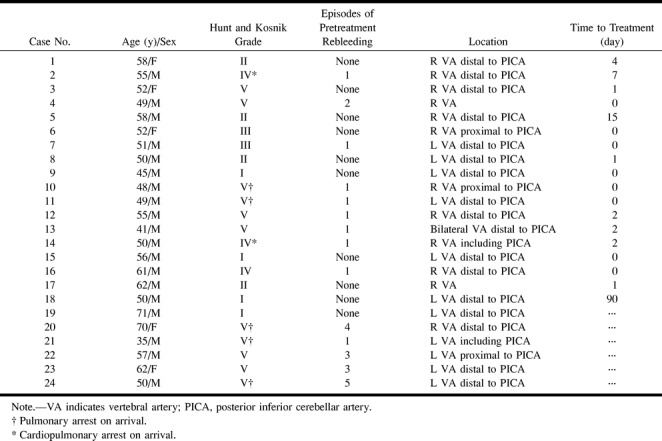
Between April 1996 and December 1999, 242 ruptured aneurysms were identified angiographically in our emergency center. Ruptured aneurysms located in the posterior fossa accounted for 56 (23%) of the cases, including 24 (10%) vertebral dissecting aneurysms. The latter were retrospectively investigated for clinical characteristics and, in 18, for efficacy of treatment. The patients ranged in age from 35 to 71 years (mean, 54 years), and the male:female ratio was 19:5 (Table 1). Preembolization neurologic function was evaluated using the Hunt and Kosnik grading system, and outcome was assessed with the Glasgow Outcome Scale (GOS). Eighteen patients (cases 1–18) were treated with platinum coils introduced via an endovascular technique to occlude the affected sites as early as possible after diagnosis. In five patients (cases 1, 3, 5, 15, and 18), whose aneurysms involved dominant vertebral arteries, balloon test occlusion preceded embolization. Two patients (cases 19 and 20) with hypoplastic contralateral vertebral arteries were followed conservatively and the other four (cases 21–24) were not eligible for treatment owing to intractable elevation of intracerebral pressure and severe brain stem dysfunction.
TABLE 1:
Clinical characteristics of patients with ruptured vertebral dissecting aneurysms
Aneurysm Location
In 12 patients, the right side was affected; in 11, the left side; and in one (case 13), the aneurysms were located bilaterally (Table 2). Seventeen were located distal to the posterior inferior cerebellar artery (PICA) origin. Only three aneurysms (cases 6, 10, and 22) were proximal and two (cases 14 and 21) included PICA involvement. In the other two (cases 4 and 17), PICA involvement was not recognized. Dominant vertebral arteries were affected in 12 patients (cases 1, 3, 5, 15, 16, and 18–24), and nondominant arteries in four (cases 4, 6, 14, and 17). In three patients (cases 15, 19, and 20), contralateral left vertebral arteries were hypoplastic, ending in the PICA. One patient (case 13) had bilateral involvement; in the other eight, the affected and contralateral arteries were almost the same size.
TABLE 2:
Outcome of patients with ruptured vertebral dissecting aneurysms treated with platinum coils at the affected site
Balloon Test Occlusion
In five cases involving dominant vertebral arteries, balloon test occlusion preceded coil embolization. A 3F Silascon balloon catheter (Kaneka Medics Co, Kanagawa) was introduced into the vertebral artery via a 6F catheter. A 6F sheath was also placed in the contralateral femoral artery. This was used to introduce a 1.8F Balt Magic microcatheter (Marincrot Medical Co, France) to infuse heparin continuously into the distal end of the balloon through a 5.5F catheter. Occlusion of the vertebral artery was verified fluoroscopically with stasis of the contrast material injected through this latter catheter. A 20-minute balloon test occlusion of the vertebral artery was carried out at the level of the body of the fifth cervical vertebra. During the 20-minute balloon test occlusion under EEG and auditory brain stem response (ABR) monitoring, heparin was infused continuously through the microcatheter and 6F and 5.5F catheters and sheaths to maintain approximately twice the control level and prevent thrombosis.
Endovascular Surgery and Techniques
Anticoagulation was used in all 18 surgically treated patients. A control activated coagulation time (ACT) was determined via a 6F sheath placed in the femoral artery. All the patients received a bolus injection of heparin (2000 U) from the sheath immediately after the measurement of control ACT. During endovascular surgery, heparin was infused continuously through a 2.5F FasTracker-18 two-marker microcatheter (Boston Scientific Corp, Watertown, MA), a 6F Envoy catheter (Cordis Endovascular Systems, Miami Lakes, FL), and the sheath to maintain ACT at 1.5 to 2.0 times the control value and prevent thrombosis (9).
Initially, a Transit soft-tip guidewire (Boston Scientific) was manipulated with the tip curved 45° in line with the parent artery. The guidewire was advanced slowly, preceding the microcatheter, to a position just proximal to the dissection site, with care taken not to enter the pseudolumen of the dissection. Thereafter, the curved guidewire was advanced slowly with a rolling motion so as not to deviate from the true lumen. Advancement of the microcatheter was performed until just proximal to the end of the dilated portion of the aneurysm. The size of the first coil was selected to match as closely as possible the maximum diameter of the aneurysm and long enough to introduce a fixed basket. Before detachment of the coils, repeat angiography was performed at 10-minute intervals to confirm fixation of the coils at the affected site. Since their introduction in Japan in May 1998, Guglielmi electrically detachable coils have been used instead of interlocking detachable coils because of their greater flexibility.
In all cases, endovascular coil embolization at the dissection site was performed as soon as possible to prevent rerupture (Table 1). In all but three cases, endovascular surgery was carried out less than 4 days after onset of symptoms. The reason for the delayed treatment in cases 2 and 18 was that the dissection was not revealed on the first angiographic study (day 0), and only became evident on day 7 in case 2 and after 3 months in case 18. In case 5, the first angiographic examination on day 0 showed a right vertebral artery dissecting aneurysm, but the contralateral left vertebral artery was not apparent until follow-up angiography on day 8. Repeat imaging on day 15 also showed persistent good blood flow so that embolization of the right vertebral dissecting aneurysm could be performed.
Follow-up angiography was performed 1 week and 1 month after embolization. MR angiography was performed 3 months, 6 months, and 1 year after treatment, usually in our outpatient clinic.
Results
Clinical Presentation and Rerupture
Nine patients presented with loss of consciousness, although eight of these were in a transient coma and recovered in less than 1 hour. In only one did loss of consciousness persist. Reports of severe neck pain were frequent, occurring in 10 patients (42%), of whom two also had shoulder pain and one had back pain. Numbness and weakness of limbs occurred in three patients each.
A clear history of hypertension was present in 11 (48%) of 23 patients, but only three had diabetes mellitus. Subsequent rupture occurred in 14 (58%) of the 24 patients (cases 2, 4, 7, 10–14, 16, and 20–24), in all except one (case 14) within 24 hours of the first attack. In eight (57%) of the patients (cases 2, 10, 11, 16, 19, 20, 22, and 23), rupture occurred within 9 hours of symptom onset; in three (21%) (cases 11, 20, and 23), it occurred immediately on arrival or en route to the hospital. Eight (57%) of the 14 patients with rerupture (cases 2, 10, 11, 13, 14, 19, 20, and 24) suffered cardiopulmonary (cases 10, 14, 19, 20, and 24) or pulmonary (cases 2, 11, and 13) arrest. All of the former fortunately recovered on resuscitation. However, in all 14 with rerupture, consciousness was disturbed: from Hunt and Kosnik grade I–II to grade III (case 4), grade IV (cases 2, 14, and 16), or grade V (10 cases). As a result of rerupture, four patients (cases 21–24) suffered a deterioration in their clinical condition. All four had intractable elevation of intracerebral pressure due to brain edema and severe brain stem dysfunction and so did not undergo aneurysmal treatment. Without embolization, in all but case 19, rerupture occurred one to five times (average, three times) between days 1 and 13 (average, day 5) until death (Table 1).
Endovascular Surgery and Complications
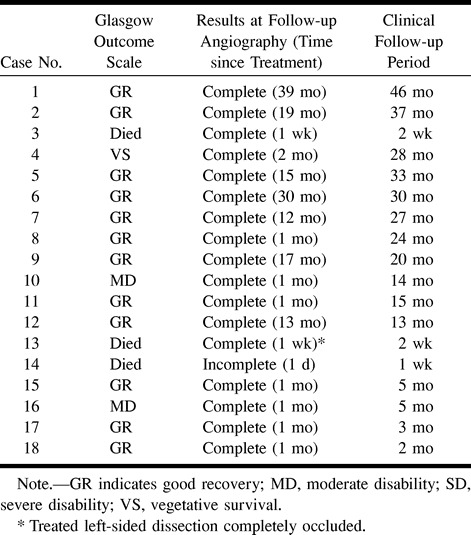
In case 4, a saccular aneurysm of the left vertebral artery was found, which was embolized before treatment of the right vertebral dissecting aneurysm. In case 13, involving the bilateral vertebral arteries at angiography on day 0, remarkable dilatation of the left dissecting aneurysm was evident in a follow-up angiogram on day 2, which was embolized to prevent rebleeding. No complications were experienced with the procedures for coil embolization. However, in case 13, rebleeding could not be prevented because of progression of the contralateral dissection, and the patient became comatose as a result of brain stem infarction. In the other 17 cases, rebleeding was not apparent (clinical follow-up ranged from 1 week to 46 months; mean, 20 months).
In case 14, with a dissection including the PICA, coil embolization was performed successfully. However, 1 day thereafter, infarction of the PICA territory occurred. Angiography performed immediately after the event showed patency of the PICA and recanalization of the vertebral artery; the cause of infarction could not be identified, although a thromboembolic event or ischemia due to the dissection was suspected. In the other 17 patients, the radiologic findings confirmed complete occlusion of the dissection site and patency of the unaffected artery (follow-up ranged from 1 week to 39 months; mean, 9 months).
Hunt and Kosnik Grade and GOS Scale
Seven patients had a Hunt and Kosnik grade of I to III. All except one (case 19, with a hypoplastic contralateral vertebral artery) were treated with coil embolization, which resulted in good recovery. Fortunately, in case 19, repeat angiography showed no morphologic change, and the clinical course was uneventful, so that discharge without neurologic deficit was possible. One year and 10 months after symptom onset, the patient was free of neurologic symptoms with no change on angiograms or MR images. Two patients had a Hunt and Kosnik grade of IV. One (case 2) made a good recovery, but the other (case 19) had cerebellar and brain stem infarction due to ischemia of the PICA territory and died. The remaining 11 had a grade of V. Six patients were treated with coil embolization and two achieved a good recovery. One other suffered moderate disability, and the other three remained in a vegetative state and died.
All five untreated patients with severe brain stem dysfunction and uncontrollable intracerebral pressure died between days 0 and 13 (mean, day 5) of repeat hemorrhage, which occurred between one and five times (average, three times).
Representative Cases
Case 2
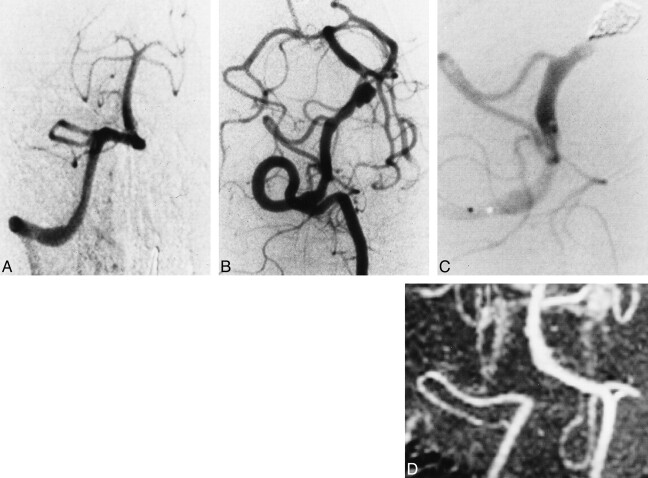
A 55-year-old man had sudden onset of a headache followed by numbness of the right limbs, at which time he sought medical attention. Six hours after the onset of symptoms, he became comatose and experienced dyspnea during examination and was immediately transported to our emergency center. Neurologic examination on admission revealed a Hunt and Kosnik grade of V, and CT scans showed marked subarachnoid hemorrhage. An emergency four-vessel angiographic study showed no abnormal findings (Fig 1A) although a second, follow-up angiogram on day 7 showed a right vertebral artery dissecting aneurysm distal to the PICA origin (Fig 1B). After successful embolization (Fig 1C), the patient recovered gradually and was discharged with no neurologic deficit 3 months after symptom onset. Follow-up angiography 2 months after embolization showed complete occlusion of the dissection site, which was confirmed on follow-up MR angiograms 7 and 19 months after the treatment (Fig 1D).
fig 1.
Case 2.
A, Right vertebral angiogram on day 1, anterolateral view, shows no abnormal findings.
B, Follow-up right vertebral angiogram on day 7 shows a dissecting aneurysm in the vertebral artery, distal to the PICA origin.
C, Right vertebral angiogram immediately after embolization of the dissection site.
D, Follow-up MR angiogram 7 months after embolization.
Case 5
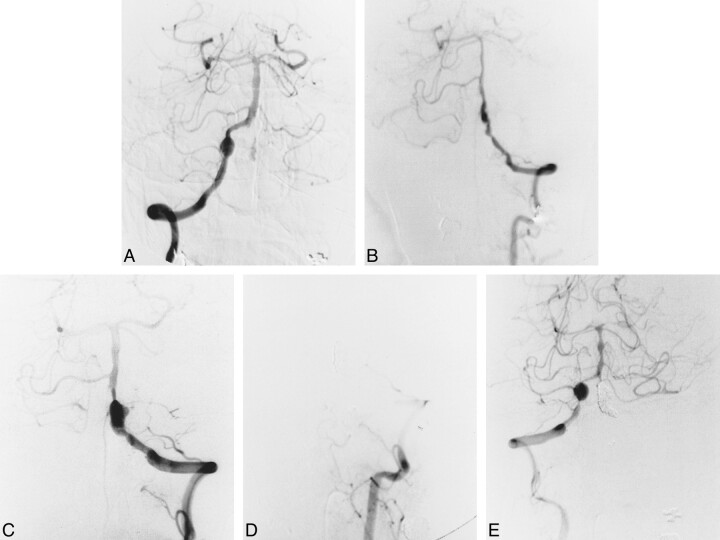
A 58-year-old man presented with sudden onset of headache and vomiting followed by persistent severe neck pain at the hospital. The severe neck pain persisted, and a neurologic examination showed a Hunt and Kosnik grade of II. CT scans revealed subarachnoid hemorrhage in the cerebellopontine angle cistern, and right vertebral angiography on day 0 showed a vertebral artery dissecting aneurysm distal to the PICA origin (Fig 2A). The left vertebral artery could not be imaged by subclavian artery angiography, and conservative treatment was recommended. A second angiogram, obtained on day 8, showed recanalization of the left vertebral artery, with a mild stenotic origin. The possibility of bilateral dissection remained, but a third angiogram on day 15 showed persistence of blood flow and normal morphology of the left vertebral artery. Coil embolization for the right vertebral artery dissecting aneurysm was therefore advocated. A preceding balloon test occlusion of the right vertebral artery showed good retrograde blood flow, with a mean arterial stump pressure of 100 mm Hg (postocclusion mean arterial pressure [100 mm Hg] / preocclusion mean arterial pressure ratio: 91%). Repeat angiography after coil detachment showed no neurologic involvement at the dissection site (Fig 2B). The patient's clinical course after embolization was uneventful and he was discharged home with no neurologic deficit. Follow-up angiography 1 month after treatment showed complete occlusion of the dissection site (Fig 2C) and patency of the contralateral left vertebral artery (Fig 2D), confirmed by follow-up MR angiography 15 months after treatment.
fig 2.
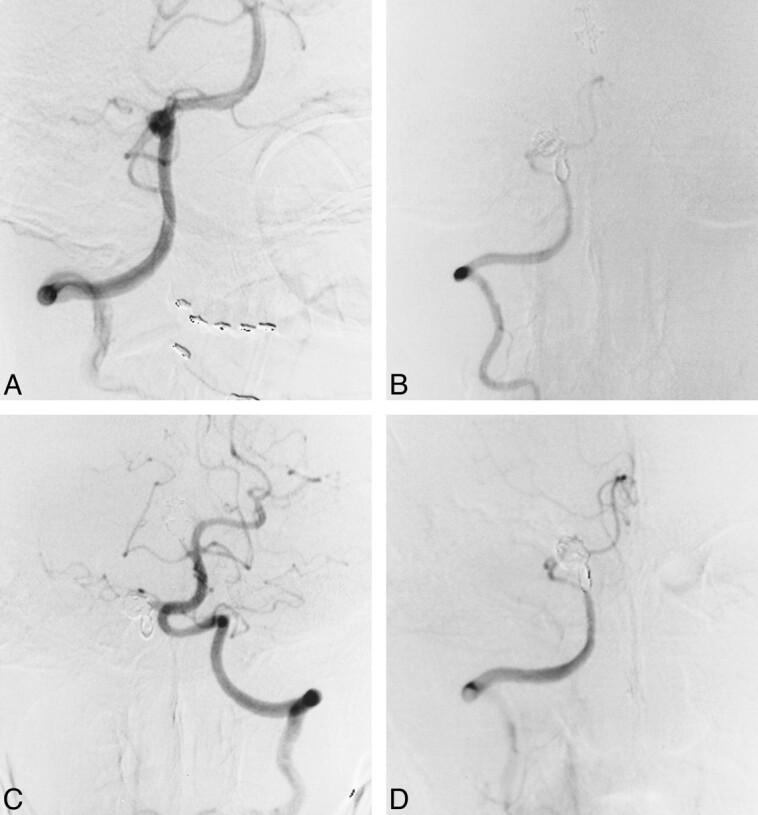
Case 5.
A, Right vertebral artery angiogram, anterolateral view, shows a dissecting aneurysm distal to the PICA origin.
B, Right vertebral angiogram, anterolateral view, immediately after coil embolization of the dissection site.
C, Left vertebral angiogram, anterolateral view, shows an increase in diameter relative to that before embolization.
D, Follow-up right vertebral angiogram, anterolateral view, 1 month after embolization shows complete occlusion of the affected site and preservation of the PICA.
Case 13
A 41-year-old man became suddenly comatose, but consciousness returned after 30 minutes while en route to our emergency center. CT on admission showed subarachnoid hemorrhage located ventral to the brain stem and the fourth ventricle. Vertebral angiography revealed a bilateral vertebral artery dissection (Fig 3A and B), which was treated conservatively. However, apnea developed after 22 hours, and a repeat CT scan showed increased hemorrhage. A second angiogram on day 2 showed marked dilatation of the left vertebral artery dissecting aneurysm (Fig 3C), prompting coil embolization (Fig 3D). A follow-up angiogram on day 10 showed progressive dissection of the contralateral right vertebral artery (Fig 3E), which resulted in cerebellar and brain stem infarction. The patient died 17 days after symptom onset.
fig 3.
Case 13.
A–E, Initial right (A) and left (B) vertebral angiograms on day 0 show bilateral vertebral artery dissection. Angiograms on day 2 show marked dilatation of the left vertebral artery dissecting aneurysm before (C) and after (D) coil embolization. Follow-up right vertebral angiogram on day 10 (E) shows progressive dissection.
Discussion
The exact rate of occurrence of dissecting vertebral artery aneurysms with subarachnoid hemorrhage is unknown; however, this condition is clearly not rare. Yamaura et al (1) reported that 24 (28%) of their 86 patients with vertebral or branch artery aneurysms had dissecting aneurysms; 21 of these patients presented with subarachnoid hemorrhage, and the other three with ischemia. In the present series, which covered a period of more than 3 years, 20 (42%) of the 48 vertebrobasilar ruptured aneurysms treated were of the dissecting vertebral artery type, constituting 10% of all cases defined by angiography in this period. Halbach et al (10) reported that two of their nine patients with subarachnoid hemorrhage and vertebral artery dissecting aneurysms had severe cardiac disturbances. In our series, cardiopulmonary or pulmonary arrest was recognized in eight (40%) of the 20 patients with ruptured aneurysms, all of which were the result of early rerupture. Fortunately, however, resuscitation could be done immediately, and recovery thereby achieved. However, only those alive on arrival were included and examined angiographically. The frequency may be an underestimate, because subarachnoid hemorrhage around the brain stem induces cardiopulmonary dysfunction, resulting in rapid death. Aoki et al (3) first reported two cases of dissecting vertebral artery aneurysms in the acute stage of rerupture. Mizutani et al (2), in a large series of 42 cases of ruptured dissecting vertebral artery aneurysms, described a high rate of rebleeding (30 cases, or 71.4%), the majority with subsequent rupture within 24 hours (17 cases, or 56.7%). A high mortality was associated with rerupture (46.7%) as compared with 8.3% without rerupture. In this series, rerupture occurred in 13 (65%) of 20 patients, all except one within 24 hours. To prevent rerupture, definitive treatment should be performed immediately after the diagnosis.
Age, Sex, and Risk Factors
Rupture of vertebral artery dissecting aneurysms frequently occurs in relatively young men (11–13) who lack atherosclerotic factors (11), as compared with conventional saccular aneurysms (14). Therefore, the pathogenesis of most dissecting aneurysms remains obscure (4). The reported mean age of occurrence ranges from 35 (11) to 53 (12) years, corresponding to ours. Male predominance of 60% to 67% has been documented in the literature (1, 12), but in the present series it was even more remarkable, at 75%. Hypertension is reportedly rare (19%) (11) or not usually present (29%) (1), but, again, that differed in our series, with about half the patients (11/23) having a verified history of hypertension.
Clinical Symptoms
Transient coma with recovery within 1 to 24 hours is an important characterizing symptom of vertebral artery dissecting aneurysms, having been reported in 11 (26%) of 42 patients in one series (2) and in seven (33%) of 21 patients in another (1). This is in line with the rate of occurrence in our series, in which eight (33%) of 24 patients experienced transient coma, all of whom recovered in less than 1 hour.
Another important initial symptom appears to be persistent severe neck pain, along with cranial nerve palsies, dysesthesia, and weakness of the limbs. In a series by Yamaura et al (1), seven of 24 patients had cranial nerve palsies; three each involving the sixth and ninth through 10th nerves, and the others involving the seventh nerve. In this series, only palsies of the sixth nerve were observed in four of the 24 patients, and all were reversible with complete recovery.
Aneurysm Location
We found no side dominance in this series, in contrast to the right-sided dominance reported by others (2, 12). Mizutani et al (2) found that in 19 (61%) of 31 cases involving one vertebral artery, the aneurysm was located in the vertebral artery distal to the PICA origin (post-PICA type); in seven, the aneurysm was in the vertebral artery involving the PICA origin (PICA-involved type); and only in five (14%) was the aneurysm in the vertebral artery proximal to the PICA origin (pre-PICA type), which was similar to our series, in which the respective values were 78%, 8%, and 14%. As to size dominance of the affected vertebral artery, the few reports in the literature (2) describe findings similar to ours: the dominant vertebral artery was affected in eight cases, the same size artery in eight cases, and the nondominant artery in three cases.
Radiologic Findings
As reported above, the initial angiogram may not always show a dissection, which leads to misdiagnosis if repeat angiography is not performed. Nohjoh et al (15) also reported one case of a dissecting vertebral artery aneurysm that was not demonstrated 1 day after subarachnoid hemorrhage but that was revealed by a second angiographic study performed 9 days later. These authors emphasized that this type of aneurysm should be suspected when the cause of the subarachnoid hemorrhage is not clear on the first angiogram. We also experienced such a case (patient 2). The finding of significant morphologic change over several days strongly suggests the existence of a dissecting aneurysm.
Treatment
Proximal occlusion of the parent vertebral artery by use of an endovascular technique or clipping may be useful for the treatment of dissecting aneurysms (4, 16–18). However, such treatment does not always completely prevent rerupture, because blood flow may persist from the contralateral vertebral artery or the thyrocervical trunk (5, 14). Some instances of growth (5) or rebleeding (3, 4, 6–8) after proximal occlusion of the parent artery have been reported. When the dissection site is located in the vertebral artery proximal to the PICA division, proximal occlusion may be effective (19), since retrograde blood flow from the contralateral vertebral artery will supply the PICA beyond this site. However, antegrade blood flow from the thyrocervical trunk may participate to preserve the vertebral artery flow. Nakai et al (6) reported rebleeding in such a situation. This is important, since the majority of vertebral artery dissections are of the post-PICA or PICA-involving type. Trapping surgery may be more reliable to prevent rerupture; however, this is invasive, and may precipitate the Wallenberg syndrome (8) or catastrophic events (2). Detachable platinum coil embolization at the dissection site has been reported (10, 20), and this may be more effective than proximal occlusion for ruptured vertebral dissecting aneurysms because of immediate cessation of the blood flow to the affected site. Coil embolization is a noninvasive technique, as compared with trapping surgery, with the further advantage of allowing prior occlusion testing.
With regard to technical assessment during coil placement, Graves et al (17) reported the utility of temporary proximal flow arrest using a nondetachable balloon, which may reduce the risk of distal emboli and preclude difficulties in deploying coils in the arterial flow stream. On the other hand, Barr et al (18) reported that adequate selection of the first coil (33–50% larger diameter than the diameter of the parent artery) with anticoagulation eliminates the need for such flow-arrest techniques to prevent distal emboli and inadvertent distal coil migration.
In this series, in which no flow-arrest techniques were used, embolic complications did not occur with continuous heparinization maintaining twice the control ACT during coil embolization. Before the coils were detached, repeat angiography usually was performed at 10-minute intervals to confirm fixation of the coils at the affected site. The reason we did not use flow-arrest techniques was to prevent inadvertent distal coil migration, which may occur when restarting blood flow just after deflating the proximal balloon.
Mahmood et al (21) reported that perforators can arise from vertebrobasilar arteries between approximately 14 mm proximal and 16 mm distal to the union. Special attention is required when a dissection is near this site. Before detachment of the coil, repeat neurologic and ABR examinations should be performed to verify the actual affected site.
When a bilateral dissection or a hypoplastic contralateral vertebral artery is encountered, preceding bypass surgery or stent placement to preserve the affected vertebral artery (22) will be needed before proceeding with embolization of the dissection site with detachable platinum coils.
Conclusion
A high rate of vertebral dissecting aneurysms may be expected in patients with subarachnoid hemorrhage, especially in those with early repeat hemorrhage. Detachable platinum coil embolization may be more effective than proximal occlusion for treatment of ruptured vertebral dissecting aneurysms because of immediate cessation of blood flow to the dissection site; however, in patients with bilateral dissections or hypoplastic contralateral vertebral arteries, prior bypass surgery or stent placement to preserve the artery will be needed.
Footnotes
Supported in part by Academic Frontier Project Funds from Monbushou (The Japanese Ministry of Education, Culture and Science).
Address reprint requests to Akira Kurata, MD, Department of Neurosurgery, Kitasato University, School of Medicine, Sagamihara, Kanagawa Prefecture, Japan 228.
References
- 1.Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg 1990;72:183-188 [DOI] [PubMed] [Google Scholar]
- 2.Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery 1995;36:905-913 [DOI] [PubMed] [Google Scholar]
- 3.Aoki N, Sakai T. Rebleeding from intracranial dissecting aneurysms in the vertebral artery. Stroke 1990;21:1628-1631 [DOI] [PubMed] [Google Scholar]
- 4.Friedman AH, Drake CG. Subarachnoid hemorrhage from intracranial dissecting aneurysm. J Neurosurg 1984;60:325-334 [DOI] [PubMed] [Google Scholar]
- 5.Irikura K, Miyasaka Y, Ohtaka H, Yada K, Hirose K. Dissecting aneurysm of the vertebral artery with lateral medullary syndrome: a case report, with special reference to surgical treatment. Jpn J Stroke 1989;11:133-139 [Google Scholar]
- 6.Nakai Y, Yanaka K, Meguro K, et al. Rebleeding from dissecting vertebral aneurysm after endovascular proximal occlusion: case report. Neurosurg Lett (Tokyo) 1999;9:21-24 [Google Scholar]
- 7.Takai N, Ezuka I, Sorimachi T, Kumagai T, Sano K. Vertebral artery dissecting aneurysm rebleeding after proximal occlusion: case report. Neurol Med Chir (Tokyo) 1993;33:765-768 [DOI] [PubMed] [Google Scholar]
- 8.Yasui T, Yagura H, Komiyama M, Fu Y, Nagata Y, Tamura K. Surgical treatment for ruptured dissecting aneurysms: proximal clipping vs trapping. Neurol Surg 1993;21:395-401 [PubMed] [Google Scholar]
- 9.Nagei S, Kurata A, Tanaka R, Irikura K, Miyasaka Y, Fujii K. Investigations of the dose of heparin and whole blood coagulation time during endovascular surgery. Intervent Neuroradiol 1997;3:215-217 [DOI] [PubMed] [Google Scholar]
- 10.Halbach VV, Higashida RT, Dowd CF, et al. Endovascular treatment of vertebral artery dissections and pseudoaneurysms. J Neurosurg 1993;79:183-191 [DOI] [PubMed] [Google Scholar]
- 11.Berger MS, Wilson CB. Intracranial dissecting aneurysms of the posterior circulation: report of six cases and review of the literature. J Neurosurg 1984;61:882-894 [DOI] [PubMed] [Google Scholar]
- 12.Manz HJ, Luessenhop AJ. Dissecting aneurysm of intracranial vertebral artery: case report and review of literature. J Neurosurg 1983;230:25-35 [DOI] [PubMed] [Google Scholar]
- 13.Yamaura A. Diagnosis and treatment of vertebral aneurysms. J Neurosurg 1988;69:345-349 [DOI] [PubMed] [Google Scholar]
- 14.Tukahara T, Wada H, Satake K, Yaoita H, Takahashi A. Proximal balloon occlusion for dissecting vertebral aneurysms accompanied by subarachnoid hemorrhage. Neurosurgery 1995;36:914-920 [DOI] [PubMed] [Google Scholar]
- 15.Nohjoh T, Houkin K, Takahashi A, Abe H. Ruptured dissecting vertebral artery aneurysm detected by repeated angiography: case report. Neurosurgery 1995;36:180-183 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka K, Waga Sh, Kojima T, Kubo Y, Shimizu T, Niwa Sh. Non-traumatic dissecting aneurysms of the intracranial vertebral artery: report of six cases. Acta Neurochir (Wien) 1989;100:62-66 [DOI] [PubMed] [Google Scholar]
- 17.Graves VB, Perl J II, Strother CM, Wallace RC, Kesava PP, Masaryk TJ. Endovascular occlusion of the carotid or vertebral artery with temporary proximal flow arrest and microcoils: clinical results. AJNR Am J Neuroradiol 1997;18:1201-1206 [PMC free article] [PubMed] [Google Scholar]
- 18.Barr JD, Lemley TJ. Endovascular arterial occlusion accomplished using microcoils deployed with and without proximal flow arrest: results in 19 patients. AJNR Am J Neuroradiol 1999;20:1452-1456 [PMC free article] [PubMed] [Google Scholar]
- 19.Yasui T, Yagura H, Komiyama M, et al. Therapeutic occlusion of unilateral vertebral artery for unclippable aneurysms: special reference to postoperative brainstem ischemia. Neurol Surg 1992;20:325-332 [PubMed] [Google Scholar]
- 20.Yamaura I, Tani E, Yokota M, et al. Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Guglielmi detachable coils. J Neurosurg 1999;90:853-856 [DOI] [PubMed] [Google Scholar]
- 21.Mahmood A, Dujovny M, Torche M, Dragovic L, Ausman JI. Microvascular anatomy of foramen caecum medulla oblongatae. J Neurosurg 1991;75:299-304 [DOI] [PubMed] [Google Scholar]
- 22.Markes MP, Drake MD, Steinberg GK, Norbash A, Lane B. Stent placement for arterial and venous cerebrovascular disease: preliminary experience. Radiology 1994;191:441-446 [DOI] [PubMed] [Google Scholar]