Abstract
Summary: We report a case of aggressive epithelial odontogenic ghost cell tumor arising from the mandible in a 32-year-old man. On CT and MR studies, the tumor was seen as a large, heterogeneous soft-tissue mass that caused marked destruction of the mandible and invaded the mouth floor and tongue base. The tumor displayed a variety of densities and signal intensities on CT and MR images, which correlated well with the degree of cellularity of epithelial islands, abundance of ghost cells and eosinophilic materials, calcification, and cystic areas on histologic sections. Owing to the unpredictable biological behavior of this type of tumor, careful, long-term follow-up is highly recommended.
The epithelial odontogenic ghost cell tumor (EOGCT) is an uncommon odontogenic lesion that is closely linked histologically to the calcifying odontogenic cyst (COC) (1). Most investigators today accept that EOGCT is a neoplastic, solid tumor counterpart of COC (2). Histologically, it consists of ameloblastoma-like odontogenic epithelial proliferations infiltrating the bone and connective tissue. Ghost cells are present as well as varying amounts of dentinoid, the latter being closely associated with odontogenic epithelium (3). Numerous names have been used to describe EOGCT, reflecting diverse histopathologic characteristics and confusion about the origin and nature of the tumor (1, 4). Occasionally, EOGCT can be locally aggressive, and histologic evidence of malignant transformation has been reported.
To our knowledge, only 16 cases of aggressive or malignant EOGCT have been reported in the English-language literature (1, 2, 5–12). Although the CT appearance has been mentioned briefly in some of them (1, 10, 12), the MR imaging findings of this unusual tumor have not been described. We report the CT and MR imaging features of aggressive EOGCT arising from the mandible in correlation with histologic findings.
Case Report
A 32-year-old man presented with a painful mass in the jaw that had enlarged progressively since he had first noticed it 4 months earlier. Physical examination revealed a 7- × 6-cm hard, fixed mass in the mandible, extending from the right first molar to the left canine and associated with an anterior open bite during mouth closure. The mass protruded posteriorly to involve the mouth floor, and the tongue base was also indurated. Although focal areas of ulceration were noted in the oral mucosa, the overlying skin appeared normal. A 1.5-cm lymph node was palpated at the right submandibular area.
Conventional radiographs showed a large, poorly defined osteolytic lesion of the mandible with several foci of increased radiopacity within it. The teeth in the vicinity of the lesion were dissolved either totally or partially. Radioisotope scans showed an area of hot uptake by the mass. Clinical and radiologic examinations, including chest radiography and abdominal sonography, revealed no evidence of mass(es) elsewhere in the body. With the assumption of primary malignant tumor of the mandible, CT and MR imaging were performed to characterize the tumor and to ascertain its extent further.
CT studies of the jaw showed a large, poorly defined, lobulated, heterogeneous mass, the epicenter of which appeared to be located in the right side of the mandible (Fig 1A and B). The mass caused irregular destruction of the body of the mandible, and grew into the cheek anteriorly and the mouth floor posteriorly. Scattered foci of presumed calcification were discovered within the mass (Fig 1A). On unenhanced CT scans, major portions of the mass located more posteriorly were hyperdense, while small portions located more anteriorly were isodense to slightly hypodense relative to adjacent muscle (Fig 1A). After administration of contrast material, moderate enhancement was seen throughout the mass, with several cystic areas remaining unenhanced (Fig 1B). CT scans also showed a 2-cm oblong right submandibular lymph node enlargement.
fig 1.
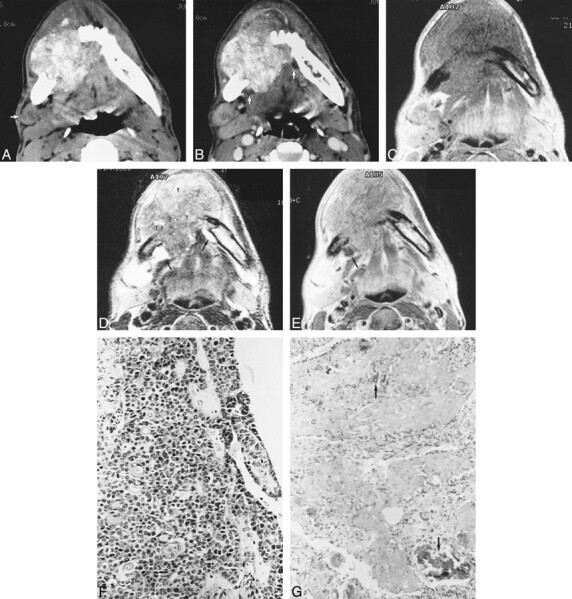
32-year-old man with aggressive epithelial odontogenic ghost cell tumor in the mandible.
A, Axial unenhanced CT scan shows a large, poorly marginated, lobulated soft-tissue mass causing irregular destruction of the body of the mandible, predominantly in the right. There are numerous calcifications varying in size within the mass. Compared with adjacent muscle, the majority of soft-tissue components of the mass show higher attenuation, while other components, mainly in the anterior aspect of the mass, show similar or slightly lower attenuation. Note lymph node enlargement (arrow) anterior to the right submandibular gland.
B, Corresponding axial contrast-enhanced CT scan more clearly shows the margin of the mass, which invades the mouth floor and tongue base. Moderate, nonhomogeneous enhancement is seen throughout the mass, with several cystic areas (arrows) remaining un[chen[chhanced. An enlarged right submandibular lymph node is also enhanced moderately, with central portions enhanced to a lesser degree.
C, Axial T1-weighted MR image shows a large soft-tissue mass destroying the mandible. Signal intensity of the mass is grossly the same as that of adjacent muscle. Also note right submandibular lymphadenopathy.
D, Corresponding axial T2-weighted MR image more clearly shows the lobulated contour and internal heterogeneity of the mass. The majority of soft-tissue components of the mass show significantly lower signal intensity than other portions, located mainly in the anterior aspect of the mass. These variations in signal intensity correlate well with density variations on corresponding unenhanced CT scan (A). Note bright signals (large arrows) from cystic components of the mass. Tiny hyperintensities (small arrows) within the mass may reflect necrotic foci or small cysts. Also note hyperintense right submandibular lymph node. Although there are several foci of small, dark signal intensities (arrowheads), calcifications are poorly seen on this MR image.
E, Axial contrast-enhanced T1-weighted MR image shows moderate, homogeneous enhancement of the mass, with cystic portions (arrow) remaining unenhanced. There is also significant enhancement of right submandibular lymphadenopathy.
F, Photomicrograph of tissue obtained from larger, posterior portion of the mass (which was hyperdense on unenhanced CT scans and hypointense on T2-weighted MR images) shows irregular islands of highly compact epithelial cells, composed primarily of small basaloid cells with hyperchromatic nuclei and scanty cytoplasm. There is prominent cellular and nuclear pleomorphism as well as evidence of frequent mitoses (hematoxylin-eosin, original magnification ×200).
G, Photomicrograph of tissue obtained from smaller, anterior portion of the mass (which was isodense or slightly hypodense on unenhanced CT scans and hyperintense on T2-weighted MR images) shows abundant ghost cell nests and eosinophilic materials containing highly basophilic foci of calcification (arrows). Epithelial cells are remarkably lacking in this area (hematoxylin-eosin, original magnification ×200).
MR studies more vividly displayed internal heterogeneity of the mass. On T1-weighted images the overall signal intensity of the mass was isointense with adjacent muscle (Fig 1C). T2-weighted images clearly showed different signal intensities from various soft-tissue portions of the mass, which correlated well with density differences on corresponding unenhanced CT scans. In general, the signal intensity of the hyperdense areas was significantly lower than that of the isodense or slightly hypodense areas on unenhanced CT scans (Fig 1D). T2-weighted MR images also showed multiple areas of cystic components in the periphery of the tumor (Fig 1D). After administration of contrast material, the entire mass showed moderate, homogeneous enhancement, with the cystic portions remaining unenhanced (Fig 1E). Although MR images more clearly depicted the mandibular destruction, mouth floor and tongue base invasion, and submandibular lymphadenopathy, they failed to show discrete evidence of calcifications seen clearly on the CT scans.
The patient underwent wide resection of the mandible combined with total glossectomy and bilateral functional neck dissection, followed by reconstructive surgery of the mandible and mouth floor. Grossly, we found a 7.6- × 6.5- × 5.5-cm relatively well demarcated, globular, soft-tissue mass that destroyed the mandible from the right first molar to the left canine. The tumor invaded the mouth floor and involved small portions of the tongue base. The cut surface of the mass was yellowish white and granular in appearance with foci of cystic change and hemorrhage. Histologically, although most portions of the tumor were solid, there were areas of cystic components within it. The solid components of the tumor were composed of irregular islands of epithelial cells of various size and shape in a fibrous stroma. While most of these islands consisted of small basaloid cells having round, hyperchromatic nuclei with scanty cytoplasm (Fig 1F), some consisted of cells with more squamous differentiation. The density (cellularity) of these epithelial cells varied from area to area within the tumor. Prominent cellular and nuclear pleomorphism as well as evidence of frequent mitoses were also noted in these cells. Ghost cells with eosinophilic cytoplasm were scattered as individual cells or as small clusters to large masses of cells throughout the tumor. Homogeneous, eosinophilic materials, believed to be degenerated ghost cells, were seen in the vicinity of ghost cell nests (Fig 1G). Surrounding stroma showed foreign body reaction with foreign body type giant cells. Scattered calcifications mainly associated with ghost cells and occasional necrotic foci were also seen within the tumor. Cystic components of the tumor were lined by thin epithelium of stratified basal cells and scattered ghost cells, characteristic of COCs. No metastatic deposits were found in any of the resected regional lymph nodes, including the right submandibular node. The histologic diagnosis was aggressive EOGCT. The patient has remained free of disease during a 2.5-year follow-up period.
Discussion
Since its first characterization by Gorlin et al (13) as a separate entity of an odontogenic origin, the true nature of COCs has been the subject of much controversy. The fact that not all COCs are cystic and that their biological behavior is often not compatible with a cyst has raised the question of whether COC is a cyst or a tumor (5, 14). Two organizing principles of classification of COCs have been put forward: monistic and dualistic (15). The monistic concept, best exemplified by the World Health Organization (WHO) classification (16), postulates that all COCs are neoplastic in nature, even though the majority are cystic in architecture and appear to be nonneoplastic. In contrast, the dualistic concept, favored by most researchers (2, 5–7, 14, 15), proposes that COCs contain two different entities, a cyst and a neoplasm.
Praetorius et al (14) classified COCs into two entities, a cyst (type 1) and a neoplasm (type 2). Type 1 was further subclassified as type 1A (simple unicystic), type 1B (odontome producing), and type 1C (ameloblastomatous proliferating), and the term dentinogenic ghost cell tumor was proposed for the type 2 lesion. Hong et al (5) suggested a modification of the Praetorius classification. These authors preferred the term epithelial odontogenic ghost cell tumor to dentinogenic ghost cell tumor, because the latter connotes a mesenchymal tissue origin and the production of true dentin, whereas the characteristic features of this tumor are odontogenic epithelial proliferation with some inductive activity and the formation of ghost cells. In the classification proposed by Hong et al (5), the neoplastic form of COC is subdivided into ameloblastoma ex COC, peripheral EOGCT, and central EOGCT. While the peripheral EOGCT occurs in the extraosseous gingival or alveolar mucosa, the central EOGCT occurs intraosseously. The many other names that have been proposed to describe this neoplastic variant of COC reflect its diverse histopathologic makeup and confusion about the origin and nature of the tumor. These include keratinizing ameloblastoma, calcifying ghost cell odontogenic tumor, cystic calcifying odontogenic tumor, dentinoameloblastoma, and peripheral odontogenic tumor with ghost cell keratinization (1, 4).
Histologically, EOGCTs are composed primarily of ameloblastoma-like areas and odontogenic epithelial islands with varying amounts of ghost cells showing keratinization and calcification (4, 14). The most important histologic feature of EOGCT that distinguishes it from conventional ameloblastoma and other odontogenic tumors is the presence of ghost cells and dentinoid substances (2). Ghost cells are believed to be transformed odontogenic epithelial cells, the mechanism of which is still unclear (4, 5). Although the presence of ghost cells is a defining feature for the diagnosis of EOGCT, these cells can also be observed in other tumors, such as pilomatricoma, craniopharyngioma, odontoma, and ameloblastic fibro-odontoma (2, 4). The nature of the dentinoid substance found in EOGCT is unknown. It is amorphous eosinophilic material containing widely separated cell bodies. It lacks the tubular structure of normal dentin, and appears as an irregular mass within the connective tissue adjacent to the proliferation of odontogenic epithelium (2).
Owing to the small number of cases as well as to the ambiguous descriptions of the morphology of EOGCTs reported in the literature, clinical data specific for these lesions are lacking. However, it is clear that EOGCTs are rare. While COCs constitute only about 1% of cysts of the jaw, less than 10% of these are EOGCTs (2). EOGCTs most commonly affect the region comprising the canine to first molar teeth in persons older than 50 years, with a slight male predilection. In general, the peripheral tumor is smaller and more common than the central tumor. While nonneoplastic forms of COC occur with equal frequency in the maxilla and mandible, EOGCT is more frequent in the mandible, whether peripheral or central. Expansion of the jaw, with clinically visible swelling and obliteration of the maxillary sinus or extension into soft tissues, has been seen with large and more aggressive central tumors (2, 5, 7). The peripheral tumors typically remain localized and can be treated with simple excision without recurrence. Although the central tumors are often amenable to curettage or simple excision, some tumors have been more aggressive and require wide resection of the jaw, as in the present case.
Although there is a question as to the true malignant nature of EOGCTs (1), a peculiar subtype with malignant potential—the so-called aggressive or malignant EOGCT, or odontogenic ghost cell carcinoma—has been recognized, with 16 cases reported in the English-language literature (1, 2, 5–12). This subtype can be diagnosed on the basis of histologic features, such as prominent mitoses, nuclear and cytoplasmic pleomorphism, hyperchromatism, necrosis, infiltrative growth pattern, and locally aggressive, destructive behavior (6). In their metaanalysis of published cases, Lu et al (11) reported that aggressive (malignant) EOGCT was more prevalent in Asians than in other racial groups, occurred more often in the maxilla than in the mandible, and was slightly more common in male than in female patients. Histologically, elements of a benign COC can be identified in all malignant variants, either separate from or admixed with the malignant epithelial components. The latter may consist of either small basaloid cells or large epithelial cells (11). Although malignant EOGCT is locally aggressive and frequently recurrent, distant metastasis is definitely uncommon. To date, only one case of pulmonary metastases has been reported (8).
The majority of malignant epithelial neoplasms of the jaw result from metastasis from distant primary malignancies or direct invasion by cancers of adjacent areas (6). The few remaining primary intraosseous carcinomas of the jaw are mostly of odontogenic origin and have been classified by WHO as malignant ameloblastoma, primary intraosseous carcinoma, malignant variants of other odontogenic epithelial tumors, and malignant changes in odontogenic cysts (16). The present case clearly seems to belong to the last category. Although the majority of the tumor was solid, cystic areas typical of COC were evident within the tumor.
From a radiologic standpoint, previous reports have mostly dealt with findings on conventional radiographs. Radiographically, the central EOGCT has been described as a purely radiolucent or mixed radiolucent-radiopaque lesion (5). It is unilocular or multilocular and can manifest as either a well-demarcated or poorly defined lesion (2, 7). The peripheral EOGCT causes no alteration or only mild erosion or saucerization of the cortical bones (2). Although CT findings have been mentioned briefly (1, 10, 12), detailed CT and MR imaging findings have not been described.
The tumor in this report showed characteristic findings on CT and MR studies that correlated well with histologic findings. Areas that were hyperdense on unenhanced CT scans and hypointense on T2-weighted MR images corresponded to those of marked cellularity of epithelial cell islands on histologic specimens (Fig 1A, D, and F). Areas that were isodense or slightly hypodense on unenhanced CT scans and hyperintense on T2-weighted MR images corresponded to those of less cellularity on histologic sections (Fig 1A, D, and G). The latter areas also had abundant ghost cells and eosinophilic materials. Scattered foci of presumed calcification on CT scans proved to be calcifications in and around ghost cell nests. Cystic areas on CT and MR studies corresponded histologically to areas typical of COC.
The recommended treatment for aggressive EOGCT is surgical excision followed by postoperative radiation with or without adjuvant chemotherapy (10, 12). However, the effectiveness of chemotherapy has not yet been determined (12). The biological behavior of aggressive EOGCT is unpredictable, and the overall 5-year survival rate is estimated to be 73% (11). This variability in biological behavior reflects different growth patterns from a slowly growing and locally aggressive tumor to a rapidly growing and highly invasive tumor. Although the patient in this report has been free of disease during a 2.5-year follow-up period, further careful observation is necessary, considering the high rate of local recurrence.
Conclusion
Knowledge of the various densities and signal intensities on CT and MR studies caused by various tissue components of EOGCT may provide a clue to the correct diagnosis of this unusual tumor.
Footnotes
Address reprint requests to Hyung-Jin Kim, MD, Department of Radiology, Inha University Hospital, 7-206, 3rd St, Shinheung-dong, Choong-ku, Inchon, 400-103, Korea.
References
- 1.Mc Coy BP, O Carroll MK, Hall JM. Carcinoma arising in a dentinogenic ghost cell tumor. Oral Surg Oral Med Oral Pathol 1992;74:371-378 [DOI] [PubMed] [Google Scholar]
- 2.Ellis GL. Odontogenic ghost cell tumor. Semin Diagn Pathol 1999;16:288-292 [PubMed] [Google Scholar]
- 3.Raubenheimer EJ, van Heerden WF, Sitzmann F, Heymer B. Peripheral dentinogenic ghost cell tumor. J Oral Pathol Med 1992;21:93-95 [DOI] [PubMed] [Google Scholar]
- 4.Gunhan O, Sengun O, Celasun B. Epithelial odontogenic ghost cell tumor: report of a case. J Oral Maxillofac Surg 1989;47:864-867 [DOI] [PubMed] [Google Scholar]
- 5.Hong SP, Ellis GL, Hartman KS. Calcifying odontogenic cyst: a review of ninety-nine cases with reevaluation of their nature as cysts or neoplasms, the nature of ghost cells, and subclassification. Oral Surg Oral Med Oral Pathol 1991;72:56-64 [DOI] [PubMed] [Google Scholar]
- 6.Ellis GL, Shmookler BM. Aggressive (malignant?) epithelial odontogenic ghost cell tumor. Oral Surg Oral Med Oral Pathol 1986;61:471-478 [DOI] [PubMed] [Google Scholar]
- 7.Colmenero C, Patron M, Colmenero B. Odontogenic ghost cell tumors: the neoplastic form of calcifying odontogenic cyst. J Craniomaxillofac Surg 1990;18:215-218 [DOI] [PubMed] [Google Scholar]
- 8.Grodjesk JE, Dolinsky HB, Schneider LC, Dolinsky EH, Doyle JL. Odontogenic ghost cell carcinoma. Oral Surg Oral Med Oral Pathol 1987;63:576-581 [DOI] [PubMed] [Google Scholar]
- 9.Folpe AL, Tsue T, Rogerson L, Weymuller E, Oda D, True LD. Odontogenic ghost cell carcinoma: a case report with immunohistochemical and ultrastructural characterization. Oral Pathol Med 1998;27:185-189 [DOI] [PubMed] [Google Scholar]
- 10.Alcalde RE, Sasaki A, Misaki M, Matsumura T. Odontogenic ghost cell carcinoma: report of a case and review of the literature. J Oral Maxillofac Surg 1996;54:108-111 [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Mock D, Takata T, Jordan RC. Odontogenic ghost cell carcinoma: report of four new cases and review of the literature. J Oral Pathol Med 1999;28:323-329 [DOI] [PubMed] [Google Scholar]
- 12.Kamijo R, Miyaoka K, Tachikawa T, Nagumo M. Odontogenic ghost cell carcinoma: report of a case. J Oral Maxillofac Surg 1999;57:1266-1270 [DOI] [PubMed] [Google Scholar]
- 13.Gorlin RJ, Pindborg JJ, Clausen FP, Vickers RA. The calcifying odontogenic cyst: a possible analogue of the cutaneous calcifying epithelioma of Malherbe. Oral Surg Oral Med Oral Pathol 1962;15:1235-1243 [DOI] [PubMed] [Google Scholar]
- 14.Praetorius F, Hjorting-Hansen E, Gorlin RJ, Vickers RA. Calcifying odontogenic cyst: range, variations and neoplastic potential. Acta Odonotol Scand 1981;39:227-240 [DOI] [PubMed] [Google Scholar]
- 15.Toida M. So-called calcifying odontogenic cyst: review and discussion on the terminology and classification. J Oral Pathol Med 1998;27:49-52 [DOI] [PubMed] [Google Scholar]
- 16.Kramer IRH, Pindborg JJ, Shear M. Calcifying odontogenic cyst. In: Kramer IRH, Pindborg JJ, Shear M, eds. Histological Typing of Odontogenic Tumors. 2nd ed. WHO International Histological Classification of Tumors. Berlin: Springer 1992;20-26


