Abstract
BACKGROUND AND PURPOSE: Enterovirus 71 (EV71) infection is now considered an important cause of childhood acute flaccid paralysis. The purpose of our study was to determine whether EV71-infection–related acute flaccid paralysis in infants and young children has characteristic MR imaging patterns.
METHODS: Seven infants and young children with acute paralysis of the upper or lower extremities and positive EV71 cultures underwent spinal MR studies during an outbreak of hand-foot-and-mouth disease in Taiwan in 1998.
RESULTS: Acute paralysis was observed in one upper extremity in two patients, in one lower extremity in three patients, and in both lower extremities in two patients. None of the patients had sensory impairment or bulbar palsy. MR studies showed unilateral or bilateral hyperintense lesions in the anterior horn regions of the cord on T2-weighted images in six patients. No abnormal signal was present in one patient. Two of three patients who received intravenous injections of contrast material had ventral root enhancement on T1-weighted images. One of them also had enhancement of the unilateral anterior horn cells. At clinical follow-up, both patients with bilateral anterior horn abnormalities had residual motor weakness, whereas only one of the five patients with unilateral involvement had residual weakness.
CONCLUSION: EV71 radiculomyelitis tends to be unilateral and to specifically involve both the anterior horn cells of the cord and the ventral roots. MR imaging allows early detection of spinal cord and root lesions.
Enterovirus 71 (EV71) infection is an emerging epidemic disease associated with acute neurologic disorders, such as aseptic meningitis, rhombencephalitis, and acute flaccid paralysis (AFP) (1–5). The recent EV71 outbreaks in Peninsular Malaysia and Taiwan affected thousands of young children (1, 2, 5), who often presented with hand-foot-and-mouth disease (HFMD) or herpangina. Most of them had a benign course; however, acute neurologic complications, mostly rhombencephalitis, can develop in some of these affected children 2 to 4 days after the onset of HFMD or herpangina (1–6). The MR imaging characteristics of EV71-related brain stem encephalitis have been reported (1, 4). EV71 infections have also been associated with poliomyelitis-like paralysis in several outbreaks worldwide since 1975 (1, 7–17), and EV71 is considered one of the leading causes of AFP now that poliomyelitis has been nearly eradicated. The MR characteristics of the spinal cord lesions in patients with AFP and EV71 infection have not, however, been described in detail (1, 4). Moreover, because the AFP caused by EV71 is usually benign compared with that caused by poliovirus, differentiating EV71-related AFP from AFP due to other causes might have important epidemiologic and therapeutic implications. Since laboratory confirmation of EV71 infection by cell culture is not always possible in the acute stage of the disease, questions may arise as to whether an MR study can provide useful information for the differential diagnosis.
We report the characteristic MR findings in seven infants and young children with culture-proved EV71 radiculomyelitis at acute and convalescent stages and correlate these findings with neurologic symptoms and clinical outcome.
Methods
Patients
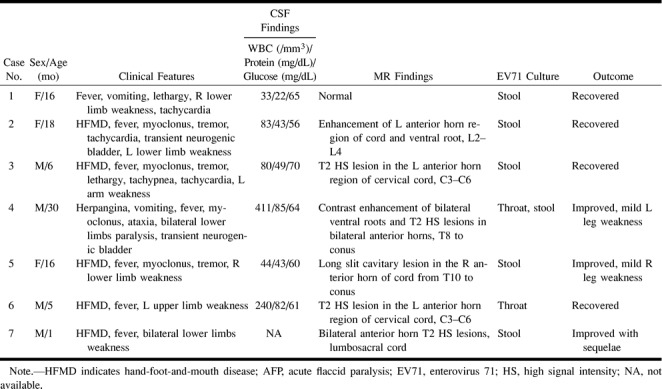
From April to December 1998, 51 infants and young children with culture-proved epidemic EV71 infection and neurologic complications were treated at our institutions. Seven of the patients (age range, 1–30 months; mean age, 13 months) were enrolled for MR study. These patients presented with biphasic pictures of AFP (defined as the acute onset of flaccid limbs and absent reflexes) following a 2- to 4-day period of prodrome with fever and/or HFMD or herpangina (small oropharyngeal vesicles and ulcers that were located chiefly on the anterior tonsillar pillars, soft palate, uvula, and pharyngeal wall). Because the remaining 44 patients had primarily signs and symptoms of brain stem encephalitis, spinal MR imaging was not performed. The spinal MR studies were obtained during the acute stage in five patients and during the convalescent stage in two. The short-term outcome and MR findings in four of the seven patients have been described elsewhere (1). EV71 was isolated from a stool specimen in six patients and from throat swabs in one. CSF was obtained in six patients for analysis, and the mean levels (±SD) of white blood cell counts, protein, and glucose were 149 ± 148 cells/mm3, 54 ± 25 mg/dL, and 63 ± 5 mg/dL, respectively (Table). The method of EV71 virus isolation has been described previously (1). In short, specimens were inoculated onto monolayers of A549 cells, green monkey kidney cells, and Vero cells. After replacement of the maintenance medium, the cells were incubated at 37°C and inspected daily for viral cytopathic effect. Isolates producing typical enteroviral cytopathic effects but untypeable with type-specific antiserum pools were typed by immunofluorescence technique with EV71 monoclonal antibodies 3323 and 3324 (Chemicon International, Temecula, CA). These isolates were further confirmed by neutralization test with polyclonal antibodies against EV71.
Clinical characteristics, CSF and neuroimaging findings, and outcome in seven young children with enterovirus 71 infection and acute flaccid paralysis
MR Studies
MR studies were performed on 1.5-T superconducting magnets. In five patients, MR examinations were performed within 2 days after the onset of AFP; the other two patients had MR examinations 2 and 7 months, respectively, after ictus. The MR sequences (depending on the scanners available) consisted of T1-weighted images with parameters of 600–750/15–20/2–3 (TR/TE/excitations), 3-mm slice thickness on sagittal images and 4- to 5-mm slice thickness on axial images; spin-echo T2-weighted images (1800–2300/90–103/1, 3-mm slice thickness) or T2-weighted fast spin-echo images (4000/85–120/1–3, 4–5-mm slice thickness); and axial gradient-echo T2-weighted images (600–808/15–25, 4–5-mm slice thickness, and 20–25° flip angle). Intravenous gadopentetate dimeglumine was administered in three patients.
The spinal segment on which the MR images were localized was predetermined by the clinically suspected lesion levels in the cord. Images were evaluated by three neuroradiologists with particular attention to the presence or absence of lesions in the anterior horn cells and roots, and to whether there was cord compression by the extramedullary mass.
Results
Initial Clinical Findings
Among the seven patients with EV71-related AFP, five had HFMD and one had herpangina. Four patients (cases 2–5) had mild and transient manifestations of brain stem encephalitis—including myoclonic jerks, tremor, or ataxia—before the onset of acute paralysis. The extent of acute paralysis included unilateral weakness involving the upper or lower extremities and bilateral weakness in the lower extremities. Two patients had unilateral arm paralysis (cases 3 and 6), three had unilateral leg weakness (cases 1, 2, and 5), and two had bilateral lower limb paralysis (cases 4 and 7). Two patients had transient urinary retention (cases 2 and 4). None of the patients with AFP had sensory impairment or signs of bulbar palsy.
Spinal MR Findings
The MR findings of EV71 radiculomyelitis and their correlative clinical characteristics are listed in the Table (page 201). Two patients (cases 3 and 6) with unilateral paralysis of the left upper extremity had hyperintense lesions in the ipsilateral anterior horn cell regions that extended variously from C3 to C7 on T2-weighted images. A contrast-enhanced lesion of the anterior horn together with ipsilateral enhancement of the ventral root of the left lumbosacral segment of the spinal cord was seen on T1-weighted images in one patient with left lower extremity paresis and transient neurogenic bladder (case 2) (Fig 1). Another patient (case 4) with bilateral lower limb weakness had hyperintense lesions bilaterally in the anterior horn regions on T2-weighted images and striking bilateral enhancement of the ventral roots on T1-weighted images after intravenous injection of gadopentetate dimeglumine (Fig 2). This patient had persistent bilateral lower limb weakness at follow-up. Residual bilateral lower limb weakness was noted in the other patient (case 7) who had bilateral anterior horn hyperintensity at the lumbosacral cord on T2-weighted studies obtained during the convalescent stage. A slitlike cavity at the anterior horn level in the right lower thoracic to lumbosacral segment of the cord was seen in one patient (case 5) (Fig 3) in whom the first MR study was performed 2 months after acute paralysis. The final patient (case 1) with right leg weakness had normal MR findings. Two of the three patients who had intravenous administration of gadopentetate dimeglumine (cases 2 and 4) had enhancement of the anterior horn regions and ventral roots on T1-weighted images; both these patients had transient neurogenic bladder. None of the patients with AFP had enhancement over dorsal roots of the involved spinal cord.
fig 1.
Unilateral AFP and transient urinary retention in 18-month-old girl with lumbosacral radiculomyelitis.
A, Contrast-enhanced axial T1-weighted image (752/15/1) at L1 level shows strong enhancement of the left ventral root (arrowhead) and mild enhancement of the left anterior horn cells (arrow) of the sacral cord.
B, Left anterior horn lesion (arrow) is inconspicuous on gradient-echo T2-weighted image (808/18/20).
fig 3.

Persistent weakness of right lower limb 2 months after EV71 infection in a 16-month-old infant.
A, Axial fast spin-echo T2-weighted image (4000/80/3) at lumbosacral cord 2 months after acute paralysis shows a hyperintense lesion in the right anterior horn region (arrow).
B, Sagittal fast spin-echo T2-weighted image shows a long-segment hyperintense lesion (arrowheads) extending from the lower thoracic to the lumbosacral levels.
Patient Outcome
After a mean neurologic follow-up period of 14 months, four patients (cases 1, 2, 3, and 6) made a complete recovery and three patients (cases 4, 5, and 7) had improved but were left with mild unilateral motor weakness. Both patients with bilateral anterior horn involvement had residual motor weakness, whereas only one of the five patients with unilateral involvement had residual motor weakness.
Discussion
EV71 infection was first identified during a small outbreak in California between 1969 and 1972 (18). Since then, several epidemics of EV71 infection have occurred around the world (1, 5, 7–17). Epidemic EV71 infections have been associated with a variety of neurologic complications, but most reports have emphasized the findings of brain stem encephalitis (1, 4, 8, 10, 11, 14, 16, 17). Sporadic cases of AFP have been associated with these EV71 outbreaks, with varying rates of incidence reported: 2% of EV71 encephalomyelitis in an outbreak in Japan (1973) (11), 7.4% in Bulgaria (1975) (10), 17% in New York (1977) (12), 4% in Hungary (1978) (8), 9% in the United States (1985–1989) (16), 58% in Brazil (1988–1990) (17), and 10% in Taiwan (1998) (1). EV71 infection will become one of the important causes of AFP after the worldwide eradication of poliomyelitis. Both EV71 and poliovirus infections affect primarily young children and are clinically similar in their AFP presentations (1). However, in the Taiwan epidemic (among the population reported here), four (57%) of the seven patients with AFP had myoclonus and tremor or ataxia, and six (86%) had HFMD or herpangina, which are rarely seen in poliovirus infections. In contrast to poliovirus-related AFP, our patients with EV71-related AFP experienced a good outcome: four (57%) of the seven patients completely recovered and three (43%) were left with mild residual weakness at more than 1 year of follow-up.
A histologic diagnosis of the causes of AFP is usually not possible in self-limited and reversible cases. In Malaysia's outbreak, four autopsy cases showed widespread inflammation in the brain stem and spinal cord, mainly in the gray matter (4). The inflammatory changes included perivascular cuffing, predominantly by mononuclear cells; neuronal degeneration and necrosis with neuronophagia; and the presence of conspicuous microglial nodules. Similar pathologic findings were also observed in two autopsy cases in Taiwan's epidemic (1, 5). Viral cultures were obtained from the specimens of spinal cord, suggesting direct invasion of EV71 into the spinal cord as the cause of AFP.
The T2 hyperintense lesions in the regions of the anterior horn cell of the spinal cord (mostly unilateral in location) explain the poliomyelitis-like paralysis in our patients. In two cases, the anterior horn lesions were accompanied by ventral root enhancement on T1-weighted MR images after intravenous contrast administration (Fig 3). The ventral root enhancement is in keeping with clinical findings of transient urinary retention. Moreover, none of our patients had sensory impairment, which coincided with the observation that none had dorsal root enhancement on MR studies. Such findings have not been described previously.
Our study suggests that the extent of MR findings in EV71-related AFP is linked to neurologic outcome. Four of the five patients with AFP and unilateral spinal cord involvement recovered completely, and only one was left with mild unilateral limb weakness. Both patients with AFP and bilateral anterior horn involvement had residual motor weakness. The complete recovery in four of our seven patients with AFP suggests that the anterior horn lesions are reversible in mild cases. In severe cases, the anterior horn lesions may undergo cavitary changes, as in one of our patients in whom slitlike cavitation was seen in the right anterior horn region of the lower thoracic and lumbosacral cord. These diverse clinical outcomes in patients with AFP are similar to those seen in patients with brain stem encephalitis (1, 4). Although EV71 has been isolated from the spinal cord in fatal cases, it remains unclear whether AFP in those patients who recovered was due to direct viral invasion of the cord or to demyelination caused by postinfectious immunologic reactions. Further study is needed to elucidate the causes of AFP in children with differing outcomes.
AFP could be caused by a variety of viruses, including poliovirus, coxsackievirus, Japanese encephalitis virus, echovirus, or enterovirus (19, 20). The tendency for brain stem and spinal cord involvement, the clinical biphasic picture, and the MR findings in poliomyelitis are all indistinguishable from those in EV71 infection. MR findings of symmetrical hyperintensity of the anterior horn cells of the spinal cord due to poliomyelitis have been described (21); however, unilateral anterior horn hyperintensity and contrast-enhanced motor roots, seen in our cases, have not been previously reported in cases of poliomyelitis. Thus, AFP radiculomyelitis is potentially distinguishable from poliomyelitis by the MR characteristics of unilateral cord involvement and root enhancement. The single published account of patients with Japanese encephalitis and AFP reported a tendency for unilateral cord involvement in older children (19). No imaging study was done in that series, even though electrophysiological results suggested the presence of anterior horn involvement. The MR findings of anterior spinal root enhancement in our patients with bilateral lower extremity weakness and urinary retention may suggest Guillain-Barré syndrome (acute demyelinating polyradiculopathy) (22). It has not been suggested that the combination of anterior horn lesions and ventral spinal root enhancement, as seen in our patients with AFP and EV71 infection, is related to this syndrome (23).
The negative MR result found in one patient (case 1) with acute right leg weakness who made a complete recovery could be explained by mild cord involvement by EV71. Similar results in EV71 rhombencephalitis, in which obvious clinical manifestations of rhombencephalitis were accompanied by negative MR imaging findings, were observed in Taiwan's outbreak (1, 4). A negative MR result may be a good predictor of better recovery in both EV71 rhombencephalitis and radiculomyelitis.
Conclusion
EV71 radiculomyelitis tends to be unilateral and to specifically involve anterior horn regions of the cord and ventral roots. The clinical manifestation of AFP is most likely reversible if it is unilateral. Patients with bilateral AFP and documented bilateral anterior horn lesions may have a less favorable outcome. EV71 infection should be suspected in young children with epidemic HFMD or herpangina complicated by neurologic signs of AFP in the limbs. MR imaging is particularly useful in the early detection of spinal cord and root lesions and should be helpful in excluding other surgically treatable causes of acute limb paralysis in children.
fig 2.

Radiculomyelitis causing bilateral AFP and urinary retention.
A, Unenhanced axial T1-weighted image (752/15) shows hypointense lesions (arrowheads) in the anterior horn cells of spinal cord bilaterally at T11 level.
B, Contrast-enhanced T1-weighted image at the same level as in A shows predominant enhancement of the ventral roots (arrowheads). The anterior horn cell lesions do not enhance.
C, Contrast-enhanced T1-weighted image at the conus level clearly shows the predominant ventral root enhancement. The slightly hyperintense dot at the left dorsal root region (arrowhead) is probably due to enhancement of the radicular vein.
D, The anterior horn cell lesions are hyperintense and more conspicuous on gradient-echo T2-weighted image (808/15/20) as compared with T1-weighted image (A).
E, Sagittal fast spin-echo T2-weighted image (2300/103/2) shows the extent of the anterior horn cell lesions (arrowheads) from midthoracic to conus levels.
Footnotes
Address reprint requests to Cheng-Yu Chen, MD, Department of Radiology, 8, Section 3, Ting-Chow Rd, Tri-Service General Hospital and National Defense Medical Center, Taipei, Taiwan, Republic of China.
References
- 1.Huang CC, Liu CC, Chang YC, et al. Neurologic complications in children with enterovirus 71 infection. N Engl J Med 1999;343:936-942 [DOI] [PubMed] [Google Scholar]
- 2. Ministry of Health, the Executive Yuan, Taiwan, ROC. Death among children during an outbreak of hand, foot, and mouth disease: Taiwan, Republic of China, April–July 1998. MMWR CDC Surveill Summ 1998;47:629-632 [PubMed] [Google Scholar]
- 3.Dolin R. Enterovirus 71: emerging infections and emerging questions. N Engl J Med 1999;341:984-985 [DOI] [PubMed] [Google Scholar]
- 4.Shen WC, Chiu HH, Chow KC, Tsai CH. MR imaging findings of enteroviral encephalomyelitis: an outbreak in Taiwan. AJNR Am J Neuroradiol 1999;20:1889-1895 [PMC free article] [PubMed] [Google Scholar]
- 5.Lum LCS, Wong KT, Lam SK, et al. Fatal enterovirus 71 encephalomyelitis. J Pediatr 1998;133:795-798 [DOI] [PubMed] [Google Scholar]
- 6.Chang LY, Huang YC, Lin TY. Fulminant neurogenic pulmonary oedema with hand, foot, and mouth disease. Lancet 1998;352:367. [DOI] [PubMed] [Google Scholar]
- 7.Chumakov M, Voroshilova M, Shindarov L, et al. Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch Virol 1979;60:329-340 [DOI] [PubMed] [Google Scholar]
- 8.Nagy G, Takatsy S, Kukan E, Mihaly I, Domok I. Virological diagnosis of enterovirus type 71 infections: experiences gained during an epidemic of acute CNS diseases in Hungary in 1978. Arch Virol 1982;71:217-227 [DOI] [PubMed] [Google Scholar]
- 9.Blomberg J, Lycke E, Ahlfors K, Johnsson T, Wolontis S, von Zeipel G. New enterovirus type associated with epidemic of aseptic meningitis and/or hand, foot, and mouth disease. Lancet 1974;2:112. [DOI] [PubMed] [Google Scholar]
- 10.Shindarov LM, Chumakov MP, Voroshilova MK, et al. Epidemiological, clinical and pathomorphological characteristics of epidemic poliomyelitis-like disease caused by enterovirus 71. J Hyg Epidemiol Microbiol Immunol 1979;23:284-295 [PubMed] [Google Scholar]
- 11.Ishimaru H, Nakano S, Yamaoka K, Takami S. Outbreaks of hand, foot and mouth disease by enterovirus 71: high incidence of complication disorders of central nervous system. Arch Dis Child 1980;55:583-588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chonmaitree T, Menegus MA, Schervish-Swierkosz EM, Schwalenstocker E. Enterovirus 71 infection: report of an outbreak with two cases of paralysis and a review of the literature. Pediatrics 1981;67:489-493 [PubMed] [Google Scholar]
- 13.Melnick JL. Enterovirus type 71 infections: a varied clinical pattern sometimes mimicking paralytic poliomyelitis. Rev Infect Dis 1984;6:S387-S390 [DOI] [PubMed] [Google Scholar]
- 14.Gilbert GL, Dickson KE, Waters MJ, Kennett ML, Land SA, Sneddon M. Outbreak of enterovirus 71 infection in Victoria, Australia, with a high incidence of neurologic involvement. Pediatr Infect Dis J 1988;7:484-488 [DOI] [PubMed] [Google Scholar]
- 15.Hayward JC, Gillespie SM, Kaplan KM, et al. Outbreak of poliomyelitis-like paralysis associated with enterovirus 71. Pediatr Infect Dis J 1989;8:611-616 [DOI] [PubMed] [Google Scholar]
- 16.Alexander JP Jr, Baden L, Pallansch MA, Anderson LJ. Enterovirus 71 infections and neurologic disease: United States, 1977–1991. J Infect Dis 1994;169:905-908 [DOI] [PubMed] [Google Scholar]
- 17.Takimoto S, Waldman EA, Moreira RC, et al. Enterovirus 71 infection and acute neurological disease among children in Brazil (1988–1990). Trans R Soc Trop Med Hyg 1998;92:25-28 [DOI] [PubMed] [Google Scholar]
- 18.Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis 1974;129:304-309 [DOI] [PubMed] [Google Scholar]
- 19.Solomon T, Kneen R, Dung NM, et al. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet 1998;351:1094-1097 [DOI] [PubMed] [Google Scholar]
- 20.Muir P, van Loon AM. Enterovirus infections of the central nervous system. Intervirology 1997;40:153-166 [DOI] [PubMed] [Google Scholar]
- 21.Malzberg MS, Rogg JM, Tate CA, Zayas V, Easton JD. Poliomyelitis: hyperintensity of the anterior horn cells on MR images of the spinal cord. AJR Am J Roentgenol 1993;161:863-865 [DOI] [PubMed] [Google Scholar]
- 22.Byun WM, Park WK, Park BH, et al. Guillain-Barré syndrome: MR imaging findings of the spine in eight patients. Radiology 1998;208:137-141 [DOI] [PubMed] [Google Scholar]
- 23.Iwata F, Utsumi Y. MR imaging in Guillain-Barré syndrome. Pediatr Radiol 1997;27:36-38 [DOI] [PubMed] [Google Scholar]



