Abstract
BACKGROUND AND PURPOSE: Because they are not well established, we investigated the technical success and recurrence rates of n-butyl 2-cyanoacrylate (NBCA) embolization of spinal dural arteriovenous fistulae (SDAVF), and assessed clinical outcomes.
METHODS: We retrospectively studied all patients with SDAVF treated by NBCA embolization at our institution over an 8-year period. Gait and micturition disabilities were analyzed. Follow-up periods averaged 3.1 years (range, 1 month to 8.9 years).
RESULTS: NBCA embolization was feasible in 74% (20/27) of patients. Of 20 patients who underwent embolization, initial embolization failure occurred in two (10%) and fistula occurrence in three (15%). All five patients in whom NBCA embolization failed underwent surgery. All patients who underwent embolization had either improved (55%) or unchanged (45%) gait disability at last follow-up. Seventeen (85%) patients had improved (40%) or unchanged (45%) micturition disability, but three (15%) had worsened. Mean Aminoff gait disability grade significantly decreased at last follow-up (2.4 [1.4] average [SD] vs 3.2 [1.4] [P = .0008]). Mean micturition disability grade decreased, but not significantly (1.4 [1.0] vs 1.7 [1.1] [P = .28]).
CONCLUSION: NBCA embolization of SDAVF was technically feasible in 75% of patients. Initial apparent successful embolization was achieved in 90%; the fistula recurrence rate (failure to occlude the draining vein) for NBCA was 15%. Comparing favorably to surgical series, NBCA embolization of SDAVF appears efficacious, significantly improving mean gait disability by almost one grade at last follow-up. Close clinical and angiographic surveillance is mandatory. Longer and more uniform follow-up is needed to determine if clinical improvement and stabilization after NBCA embolization are sustained.
As an alternative to the surgical treatment of spinal dural arteriovenous fistulae (SDAVF), interruption of the intradural draining vein can be achieved by endovascular embolization with liquid embolic material (1–5). Because isobutyl 2-cyanoacrylate (IBCA) (6) is no longer commonly used, n-butyl 2-cyanoacrylate (NBCA) is considered to be the optimal embolic agent for SDAVF embolization. Nevertheless, the efficacy and recurrence rates of NBCA-treated SDAVF have yet to be firmly established. Furthermore, regardless of whether surgery or endovascular embolization excludes the arteriovenous fistulae, studies investigating clinical outcome and prognostic factors of treated SDAVF are relatively limited and have been mostly descriptive (2, 4, 7–12). In particular, the reported technical experience of SDAVF embolized with NBCA and their clinical outcome assessments are scarce (1, 2, 4, 11, 13, 14). Prior series that have used IBCA (1, 4) or other embolic material (11, 13), have reported on few patients (14), have had relatively short follow-up periods, or have described limited clinical follow-up data qualitatively without disability assessment (4).
Although most clinical series have documented improvement or stability in functional status for the majority of treated patients, the longevity of treatment results and the degree to which patients are expected to improve after treatment have not been well established (8). Moreover, although it has been suggested that younger patients with shorter duration of symptoms before treatment have better outcomes (4, 8, 9, 11), it is unknown whether the extent of cord edema or ischemia and the prominence of perimedullary vessels on pretreatment MR images or the degree of their resolution or persistence on follow-up MR examinations is prognostic for functional recovery (15).
Methods
From April 1991 to March 1998, 20 patients (16 [80%] men and four [20%] women aged 47–83 years [mean, 66 years]) who had angiographically proven SDAVF with pial venous drainage underwent NBCA embolization at our institution. If NBCA embolization failed to show occlusion of the fistulae, either during initial embolization or follow-up spinal angiography, surgery was then performed. In seven additional patients, NBCA embolization was not technically feasible; these patients were treated surgically without an embolization attempt.
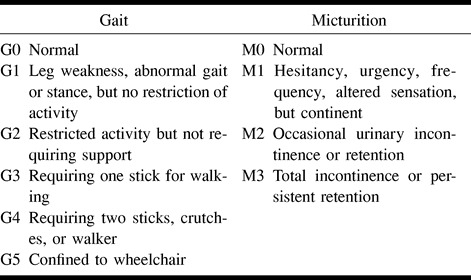
The medical records of all patients were retrospectively reviewed. Up-to-date information was gathered by outpatient review (seven treated patients) and phone questionnaire (13 treated patients). In cases of death, functional status prior to death was obtained from family members. Pre- and postintervention spinal MR examinations and follow-up spinal angiograms were retrospectively reviewed. If unavailable, information was obtained from dictated reports. Gait and micturition disabilities (Table 1) were recorded prior to any intervention and at last follow-up.
TABLE 1:
Aminoff {&} Logue's scale of disability (10)
All patients were treated under general anesthesia to minimize patient motion and optimize digital subtraction angiography (DSA) and roadmapping. If no fistula was found after selective thoracic and lumbar injections, and delayed venous filling was present at the artery of Adamkiewicz, comprehensive selective spinal angiography was performed, including injection of common cartotid, vertebral, thyrocervical, costocervical, and internal iliac arteries. When the fistula was identified, superselective catheterization was performed using a variably stiff microcatheter with the aid of digital fluoroscopy and roadmapping. DSA runs were performed to improve visualization of the fistula and to exclude the presence of anterior or posterior spinal arteries. When the microcatheter was positioned as closely to the fistula as possible, angiographic flow characteristics were analyzed to determine if NBCA could safely reach the proximal intradural draining vein. If embolization was deemed unsafe, the procedure was terminated, and an immediate surgical recommendation was made. If embolization was deemed safe, multiple test injections with contrast material were performed to gauge flow characteristics. Then, using a single-column technique, 0.2 to 0.3 cc of the 25% to 33% diluted NBCA:Ethiodol mixture was injected under direct fluoroscopic guidance. After delivery of NBCA, the microcatheter was promptly removed to prevent inadvertent catheter adhesion to the vessel. Control spinal angiography was then performed from at least two levels above to at least two levels below the shunt level bilaterally. Venous phase response of the artery of Adamkiewicz was also evaluated by use of control angiography (normal < 12 s). If the fistula persisted after embolization, surgical recommendation was made. If the patient's physical examination was worse after embolization, spinal MR imaging and another spinal angiogram were performed. If the repeat spinal angiogram revealed no residual fistula and an exceptional second fistula could not be identified, patients were treated with anticoagulants, steroids, and hypertensive volume expansion therapy. During follow-up spinal angiography, selective spinal angiography was performed.
Pre- and postintervention mean gait and micturition disability grades were compared for statistically significant differences by using the Wilcoxon rank-sum test. Pre- and postintervention gait disability change (better, same, worse categories) at last follow-up were correlated with age, duration of symptoms before treatment (before 18 months and before 30 months), preintervention disability, superior extent of abnormally high T2 signal within the spinal cord on preinterventional MR images, and degree of resolution of abnormal T2 cord signal on follow-up MR images. These correlations were statistically analyzed using Fisher's exact test probabilities. For Fisher's exact tests, contingency tables (2 × 2) were constructed to differentiate between proportions of patients who were improved versus patients who were either unchanged or worse.
Results
Clinical Data
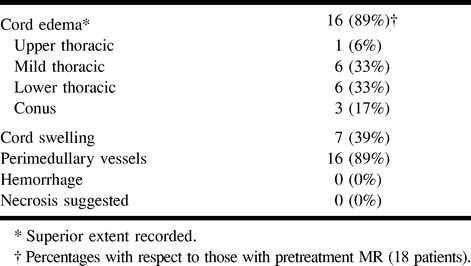
The most frequent initial symptom was leg weakness (75%) followed by sensory deficit (70%). Almost all patients had a triad of leg weakness, sensory deficit, and micturition disorder by the time of diagnosis (Table 2). The average time from initial symptoms to diagnosis was 1 year 9 months (range, 3–60 months). Eighty percent of patients had progressive symptoms, whereas 20% had both progressive and stepwise worsening. Myelograms obtained from 20% of patients suggested the diagnosis of spinal arteriovenous malformation, whereas 80% of patients underwent MR examinations that showed cord edema and/or perimedullary vessels, suggesting the diagnosis (Table 3).
TABLE 2:
Symptom distribution on initial evaluation and at time of diagnosis*
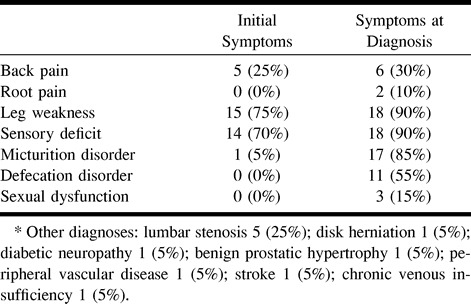
TABLE 3:
Spine MR examination findings before NBCA embolization
The most common fistula locations were T12, followed by T6, L2, and S3 (Fig 1). Twelve fistulae were on the right; nine were on the left (one patient had two concurrent fistulae at distinctly different levels).
fig 1.
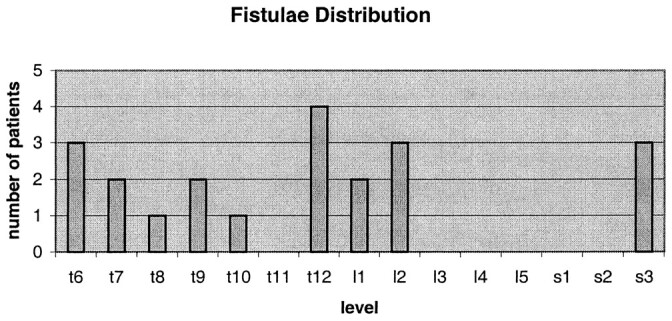
Distribution of 21 SDAVF in 20 patients treated with NBCA embolization
Treatment Data
Treatment data are summarized in Table 4. Fifteen (75%) patients were successfully treated with NBCA embolization. Five (25%) patients were treated by surgery after failed embolization. In two (10%) patients, initial embolization failed to occlude the proximal intradural draining vein completely, as documented by control spinal angiography. In three (15%) patients, the SDAVF continued to fill by collateral circulation that had enlarged or developed, as documented on follow-up spinal angiography. For the two patients with initial embolization failure, surgery was performed 2 and 13 days later, respectively. For the three patients with embolization failure and fistula recurrence, the average time from embolization attempt to surgery was 50 days (range, 13–97 days). Of the total 27 patients in whom SDAVF was confirmed by spinal angiography during the study period, embolization was technically not feasible in seven (23%) patients (Table 5), and therefore no embolization was attempted. These patients had their SDAVF treated surgically within the next 1 to 2 days.
TABLE 4:
Treatment profile of twenty patients with SDAVF embolized with NBCA
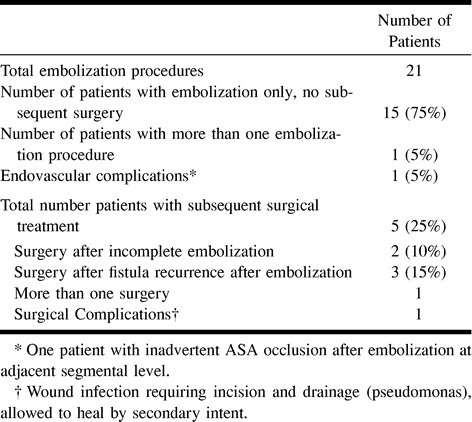
TABLE 5:
Reasons NBCA embolization technically not feasible (no embolization attempt) in seven additional patients

Follow-up Imaging Data
Twenty-one follow-up spinal angiograms were obtained from 13 (65%) patients; two follow-up angiograms were obtained from six patients each, and three were obtained from one. The average time from last treatment to initial follow-up spinal angiography was 204 days (range, 4 days to 3 years). Seventeen follow-up spinal angiograms were negative for fistula recurrence, whereas four were positive. Three (15%) fistulae recurred after NBCA embolization and one after surgery.
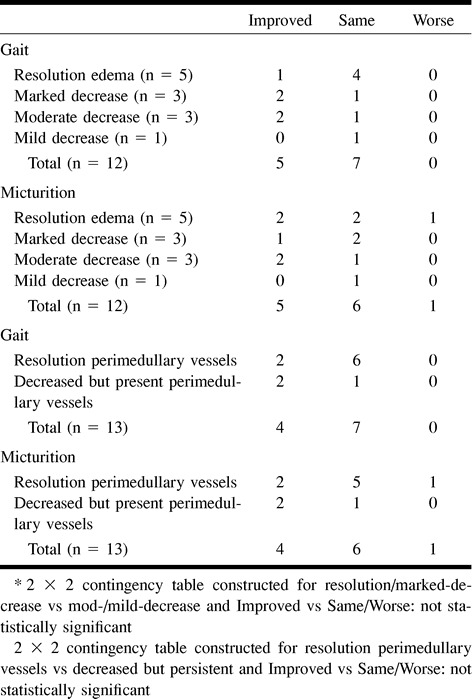
Twelve (67% of those with comparative studies) patients had both preintervention and follow-up spinal MR examinations. The average time from last intervention to follow-up MR imaging was 96 days (range, 2–357 days). All follow-up MR examinations showed some degree of decreased edema and perimedullary vessel prominence. The majority of patients (67%) had resolution or marked decrease of T2 signal intensity, indicating abnormal cord (Table 6). Perimedullary vessel resolution was documented in 73% of patients.
TABLE 6:
Imaging results of those patients with follow-up MR (12 patients)*

Clinical Outcome
The last time from latest intervention, either surgery or embolization, to most recent follow-up was 1124 days (3.1 years), ranging from 1 month to 8.9 years (at 1 month [one patient], between 1–12 months [four patients], after 12 months [15 patients]).
Comparing preintervetion and last follow-up postintervention disability, ie, clinical outcome in categorical terms, 11 (55%) patients had less gait disability, whereas nine (45%) were unchanged. No patient had worse gait disability. Therefore, all 20 NBCA-embolized (100%) patients with SDAVF had improved or unchanged gait disability at last follow-up. Overall, 17 (85%) patients had improved or unchanged micturition disability at last follow-up: eight (40%) patients were improved, nine (45%) were unchanged, and three (15%) were worse. For the NBCA embolization without surgery group (15 patients), six (40%) patients had less gait disability, whereas nine (60%) were unchanged. No patient had worse gait disability. Therefore, all 15 (100%) patients with SDAVF who underwent successful NBCA embolization (no initial failure or fistula recurrence) had improved or unchanged gait disability at last follow-up. In this group of patients, 87% showed improved or unchanged micturition disability at last follow-up: five (33%) patients were improved, eight (53%) were unchanged, and two (13%) were worse.
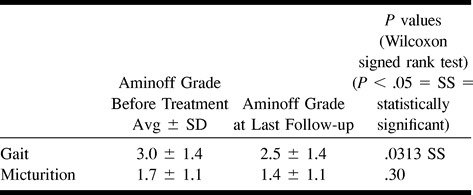
Comparing pre- and last follow-up postintervention disability in quantitative terms for all NBCA-embolized patients, mean Aminoff gait disability grade significantly improved (2.4 ± 1.4 [average ± SD] vs 3.2 ± 1.4 [P = .0008]). Mean micturition grade was decreased, but not significantly (1.4 ± 1.0 vs 1.7 ± 1.1 [P = .28] (Table 7A). For patients who underwent successful NBCA embolization that did not require subsequent surgery, mean Aminoff gait disability grade was also significantly improved at last follow-up (2.5 ± 1.4 vs 3.0 ± 1.4 [P = .0313]). Mean micturition grade was again decreased, but not significantly (1.4 ± 1.1 vs 1.7 ± 1.1 [P = .30]) (Table 7B). For patients treated by surgery after undergoing NBCA embolization (n = 5), because of small numbers, Aminoff gait disability grades did not appear to be significantly improved after treatment (2.0 ± 1.6 vs 3.6 ± 1.5 [P = .0625]), nor were micturition grades (1.4 ± 0.9 vs 1.6 ± 1.1 [P = .88]).
TABLE 7A:
Aminoff functional grades before and post intervention for all NBCA-embolized patients (n = 20)

Predictors of Outcome
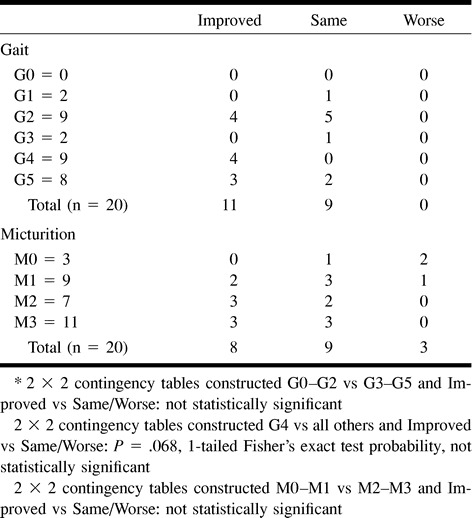
Preintervention gait disability was not significantly associated with improvement after treatment (Table 8). Patients with grade 4 gait disability tended to benefit from treatment. Preintervention micturition disability was not associated with improvement after treatment.
TABLE 8:
Disability assessment results compared with preintervention Aminoff functional grades*
No association was found between duration of symptoms for fewer than 30 months and improved clinical outcome (Table 9). For patients treated within 13 months, micturition and gait disability outcomes were not significantly improved (Table 10). No association was found between improved clinical outcome and age (Table 11) or pre- (Table 12) and postintervention cord edema or perimedullary vessel resolution on MR images (Table 13).
TABLE 9:
Disability assessment at last follow-up between groups (duration of symptoms <30 months vs ≥30 months before treatment)*
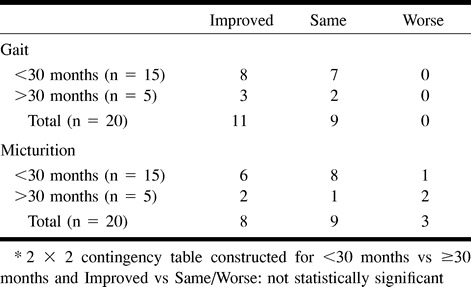
TABLE 10:
Disability assessment at last follow-up between groups (duration of symptoms <13 months vs ≥18 months before treatment)*
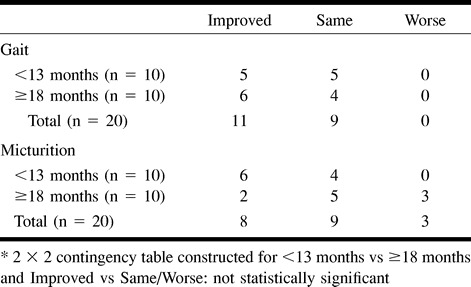
TABLE 11:
Disability assessment at last follow-up between two age groups (<65 yrs and ≥65 yrs)*
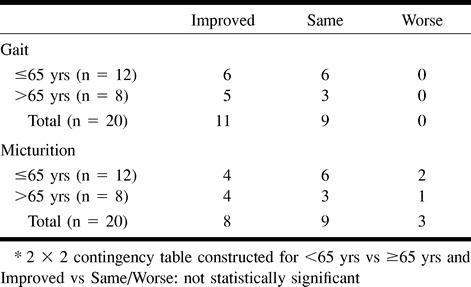
TABLE 12:
Preintervention MR superior extent of cord edema compared with clinical outcome*
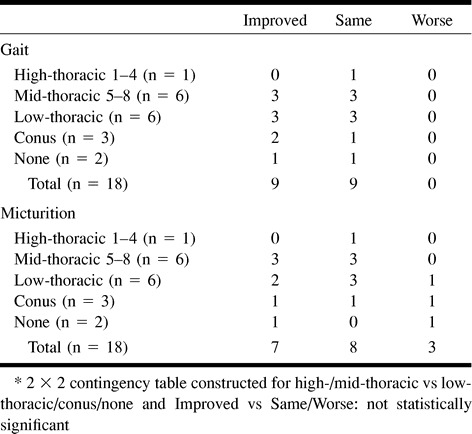
TABLE 13:
Pre- and postintervention MR cord edema and perimedullary vessel response compared with clinical outcome*
Discussion
The technical success and recurrence rates of NBCA embolization of SDAVF are better than those of embolization with polyvinyl alcohol particles (13, 16, 17), where the majority of patients will have a symptomatic recurrence, but they have yet to be established firmly (4). In the largest available series, Berenstein's experience as reported by Niimi et al (4), 30 patients with SDAVF were treated with NBCA embolization from 1988 to 1995, but only 12 patients were followed up longer than 12 months (average, 19 months). Concentrating on the technical aspects in Berenstein's series, 25 (75%) of 30 patients initially underwent “adequate” embolization with NBCA, and for those patients treated with longer than a 1-month follow-up, fistula recurrence was documented in four (18%) of 22 patients.
In our NBCA series, initial apparent “adequate” embolization, as defined by Berenstein and Niimi (4) (penetration of NBCA to the fistulae or draining vein or both; angiographic disappearance of the fistulae or their drainage after embolization [determined by bilateral angiography of two pedicles above, two pedicles below, and at the level of the shunt]; no compromise of venous drainage of the spinal cord after embolization), was achieved in 18 (90%) of 20 patients. Two (10%) patients had residual filling of their fistulae on immediate postembolization spinal angiograms and were promptly treated surgically. Three (15%) patients had angiographically verified fistula recurrence, despite initial apparent “adequate” embolization, and underwent subsequent surgery for definitive treatment. This suggests that, even in experienced hands, the initial apparent technical success rate of NBCA embolization of SDAVF is 75% to 90%, and that the recurrence rate is at least 15% to 20%. However, in our experience, when NBCA can clearly be documented to be in the draining vein after embolization, the recurrence rate is zero. If we are not confident that NBCA has occluded the draining vein, yet embolization appears “adequate,” short-term follow-up spinal angiography is recommended, and is typically performed within 1 month.
We attempted NBCA embolization in 75% of 27 consecutive patients with SDAVF (percentage unknown in Berenstein's series). One in four patients were treated surgically without an embolization attempt, most commonly as a result of an anterior spinal artery arising from the same pedicle or unfavorable small angioarchitecture, preventing safe microcatheter positioning. Currently, diagnostic catheters (eg, Simmons, Cobra) serve as guiding catheters for the microcatheters, but this is not optimal. The future development of specialized spinal guiding catheters with softer and more suitably shaped tips, as well as smaller and safer microcatheters and microguidewires, will help overcome these anatomic and technical limitations, and will likely decrease the fistula recurrence rate.
The serious complications of spinal angiography and embolization are primarily related to ischemic injury to the spinal cord. In a retrospective analysis of spinal arteriography in 96 consecutive patients, Forbes et al (18) found transient neurologic deficits in 2.2% (two patients recovering within 24 hours and 1 patient within 1 week). Niimi et al (4) described no neurologic complications in their SDAVF embolization series. One complication occurred in our series. During NBCA embolization, an anterior spinal artery occlusion occurred at an adjacent level, leading to a transient neurologic deficit. Prior to embolization, this 59-year-old man had 4+/5 motor strength of his proximal lower extremities, walked independently but with restricted activity, and had occasional bladder incontinence. The left T10 feeding fistula was occluded with NBCA; however, on subsequent control angiograms, injection of the left T9 pedicle showed that the anterior spinal artery no longer filled. The patient immediately experienced worsening lower extremity motor weakness, examined at 4/5 proximally, and the sensation of leg “heaviness.” He was promptly administered steroids and underwent anticoagulant and hypertensive/hypervolemic therapy. Posttreatment CT revealed high-attenuation material (NBCA) anterior to the cord at T9, with more high-attenuation material posteriorly at T10 and T11 at the level of the fistula. At the time of discharge, however, the patient had 5/5 motor strength and felt improved compared with his pretreatment status.
From what is known of the natural history of untreated patients with SDAVF (10), once leg weakness or disturbance of gait develops, it often progresses rapidly until the patient is severely disabled (gait grade 4 and 5). Progressive myelopathy from presumed venous hypertension (9, 19, 20) is unrelenting, with almost no possibility of spontaneous improvement. It is estimated the 50% of untreated patients will be severely disabled within 3 years of leg weakness onset (10). However, with the development of microsurgical techniques (7–9, 21, 22) and later endovascular techniques (1), treatment of SDAVF has advanced rapidly, and SDAVF are now considered curable. Unfortunately, despite advances in MR imaging and digital subtraction angiography, and increased clinical awareness of the disease, early and timely diagnosis of SDAVF remains a challenge. Therefore, although the disease is curable, the longstanding myelopathy is rarely completely reversible. Nonetheless, surgery or embolization improves or stabilizes symptom progression in most patients. It is unknown, however, whether clinical improvement after treatment is sustained in long-term follow-up (8).
All 20 patients who underwent NBCA embolization had improved (55%) or unchanged (45%) gait disability at last follow-up. The main benefit of NBCA embolization for patients disabled by SDAVF seems to be an expected improvement in gait disability. At last follow-up, mean Aminoff gait disability grades were significantly improved (2.4 ± 1.4 (average ± SD) vs 3.2 ± 1.4 [P = .0008]). Furthermore, despite severe preintervention gait disability and prolonged symptoms before treatment, some of these patients improved. These results for NBCA embolization compare favorably with the outcomes of available surgical series.
The longest follow-up study for surgically treated patients with SDAVF is Symon et al's series (9), as reported by Tacconi et al (8). Twenty surgically treated patients were followed up beyond 3 years, with follow-up ranging from 3 to 24 years. They found that at 18 to 36 months after surgery, improvement (particularly regarding gait) and stabilization of total disability (gait, micturition, and bowel control) was documented in 84% of patients. However, at last follow-up (mean, 147 months), only 35% of available patients were improved or stabilized (8). In another series, Oldfield et al's study (22) as reported by Afshar et al (7), 19 patients were followed up a mean of 37 months. They showed a significant improvement of less than one grade (0.4) in mean gait disability at last follow-up.
Tacconi et al's (8) and Symon et al's series (9) assessed overall disability without investigating its components separately. Afshar and colleagues (7) and Oldfield et al (2) did not investigate micturition disability. In our series, although 17 (85%) patients had improved (40%) or unchanged (45%) micturition disability at last follow-up quantitatively (three patients [15%] were worse), mean micturition disability grade was not significantly lowered after NBCA embolization (1.4 ± 1.0 vs 1.7 ± 1.1 [P=.28]). Although improvement in micturition occurred on an individual basis as a component of total disability, micturition disability improvement was less consistently seen after treatment. The posttreatment prognosis of SDAVF-induced micturition disability, and likely bowel disability, is worse than for gait disability.
As suggested by others (8, 9), younger age (<65 years) did not correlate with improved clinical outcome at last follow-up. Moreover, contrary to the intuitive concept that shorter duration of symptoms and better pretreatment disability status correlates with clinical improvement after treatment (4, 8, 9, 11, 22), in our patients no such correlation was found. In addition to the relatively small number of patients in the study, a further limitation was uniform follow-up could not be obtained; therefore, selection bias likely explains in part this discrepancy. Nonetheless, elderly patients with longstanding severe gait or micturition disability should not be dissuaded from surgical or embolic treatment of their SDAVF because they may benefit significantly.
Some authors have investigated a possible correlation between MR findings and clinical improvement (15). We found no correlation with clinical improvement and preintervention superior extent of abnormally high T2 signal intensity of cord, degree of resolution of abnormal cord signal on postintervention MR images, and degree of resolution of prominent perimedullary vessels on postintervention MR images. Most patients had resolution or marked decrease of abnormally high T2 signal intensity of cord on follow-up MR images. This implies that patients with treated SDAVF with clinical deterioration should undergo a follow-up spinal angiogram to exclude fistula recurrence, regardless of the MR imaging findings. Nonuniform and incomplete follow-up MR imaging was present, leading to selection bias, which could mask a significant correlation. Also, abnormal cord signal and perimedullary vessels are macroscopic manifestations of SDAVF venous hypertension and ischemia; permanent microscopic cord injury may not be evident despite the resolution of overt MR imaging signal abnormalities.
Conclusion
NBCA embolization of SDAVF was technically feasible in 75% of patients. When embolization was attempted, initial apparent “adequate” embolization was achieved in 90%. NBCA failed to occlude the draining vein in 15% of patients who underwent embolization. Comparing favorably to the follow-up results of available surgical series, successful NBCA embolization of SDAVF appears efficacious, significantly improving patients' gait disability by almost one grade at follow-up. Although NBCA embolization offers the benefits of less invasiveness and earlier rehabilitation because embolization is not technically feasible in up to 25% of patients and the recurrence rate is relatively high, close collaboration with an experienced neurovascular surgeon and vigilant clinical and angiographic follow-up are recommended. No clinical or imaging prognostic factors were found. Continued and more uniform long-term follow-up is needed to verify that improved and stabilized gait and micturition disability after NBCA embolization is lasting.
TABLE 7B:
Aminoff functional grades before and post intervention, patients with NBCA embolization only with subsequent surgery (n = 15)
Footnotes
Address reprint requests to Fernando Viñuela, MD, Professor and Director, Division of Interventional Neuroradiology, Department of Radiology, Box 951721, UCLA School of Medicine and Medical Center, 10833 Le Conte Ave, Los Angeles, CA 90095-1721.
References
- 1.Merland JJ, Riche MC, Chiras J. Intraspinal extramedullary arteriovenous fistulae draining into the medullary veins. J Neuroradiol 1980;7:271-320 [PubMed] [Google Scholar]
- 2.Mourier KL, Gelbert F, Rey A, et al. Spinal dural arteriovenous malformations with perimedullary drainage. Indications and results of surgery in 30 cases. Acta Neurochir (Wien) 1989;100:136-141 [DOI] [PubMed] [Google Scholar]
- 3.Barth MO, Chiras J, Rose M, Vega Molina J, Bories J. [Results of embolization of spinal dural arteriovenous fistulas with perimedullary venous drainage]. Neurochirurgie 1984;30:381-386 [PubMed] [Google Scholar]
- 4.Niimi Y, Berenstein A, Setton A, Neophytides A. Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery 1997;40:675-682 discussion 682–683 [DOI] [PubMed] [Google Scholar]
- 5.Gobin YP, Houdart E, Casasco A, Merland JJ. Endovascular therapy for arteriovenous malformations and fistulae in the spinal cord. Semin Intervent Radiol 1993;10:227-242 [Google Scholar]
- 6.Samson D, Marshall D. Carcinogenic potential of isobutyl-2-cyanoacrylate [letter]. J Neurosurg 1986;65:571-572 [DOI] [PubMed] [Google Scholar]
- 7.Afshar JK, Doppman JL, Oldfield EH. Surgical interruption of intradural draining vein as curative treatment of spinal dural arteriovenous fistulas [see comments]. J Neurosurg 1995;82:196-200 [DOI] [PubMed] [Google Scholar]
- 8.Tacconi L, Lopez Izquierdo BC, Symon L. Outcome and prognostic factors in the surgical treatment of spinal dural arteriovenous fistulas. A long-term study. Br J Neurosurg 1997;11:298-305 [DOI] [PubMed] [Google Scholar]
- 9.Symon L, Kuyama H, Kendall B. Dural arteriovenous malformations of the spine. Clinical features and surgical results in 55 cases. J Neurosurg 1984;60:238-247 [DOI] [PubMed] [Google Scholar]
- 10.Aminoff MJ, Logue V. The prognosis of patients with spinal vascular malformations. Brain 1974;97:211-218 [DOI] [PubMed] [Google Scholar]
- 11.Westphal M, Koch C. Management of spinal dural arteriovenous fistulae using an interdisciplinary neuroradiological/neurosurgical approach: experience with 47 cases. Neurosurgery 1999;45:451-457 discussion 457–458 [DOI] [PubMed] [Google Scholar]
- 12.Behrens S, Thron A. Long-term follow-up and outcome in patients treated for spinal dural arteriovenous fistula. J Neurol 1999;246:181-185 [DOI] [PubMed] [Google Scholar]
- 13.Morgan MK, Marsh WR. Management of spinal dural arteriovenous malformations. J Neurosurg 1989;70:832-836 [DOI] [PubMed] [Google Scholar]
- 14.Ushikoshi S, Hida K, Kikuchi Y, et al. Functional prognosis after treatment of spinal dural arteriovenous fistulas. Neurol Med Chir (Tokyo) 1999;39:206-212 discussion 212–213 [DOI] [PubMed] [Google Scholar]
- 15.Willinsky RA, terBrugge K, Montanera W, Mikulis D, Wallace MC. Posttreatment MR findings in spinal dural arteriovenous malformations. AJNR Am J Neuroradiol 1995;16:2063-2071 [PMC free article] [PubMed] [Google Scholar]
- 16.Hall WA, Oldfield EH, Doppman JL. Recanalization of spinal arteriovenous malformations following embolization. J Neurosurg 1989;70:714-720 [DOI] [PubMed] [Google Scholar]
- 17.Nichols DA, Rufenacht DA, Jack CR, Jr. Forbes GS. Embolization of spinal dural arteriovenous fistula with polyvinyl alcohol particles: experience in 14 patients [see comments]. AJNR Am J Neuroradiol 1992;13:933-940 [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes G, Nichols DA, Jack CR, Jr. et al. Complications of spinal cord arteriography: prospective assessment of risk for diagnostic procedures. Radiology 1988;169:479-484 [DOI] [PubMed] [Google Scholar]
- 19.Aminoff MJ, Barnard RO, Logue V. The pathophysiology of spinal vascular malformations. J Neurol Sci 1974;23:255-263 [DOI] [PubMed] [Google Scholar]
- 20.Aminoff MJ, Logue V. Clinical features of spinal vascular malformations. Brain 1974;97:197-210 [DOI] [PubMed] [Google Scholar]
- 21.Krayenbuhl H, Yasargil MG, McClintock HG. Treatment of spinal cord vascular malformations by surgical excision. J Neurosurg 1969;30:427-435 [DOI] [PubMed] [Google Scholar]
- 22.Oldfield EH, Di Chiro G, Quindlen EA, Rieth KG, Doppman JL. Successful treatment of a group of spinal cord arteriovenous malformations by interruption of dural fistula. J Neurosurg 1983;59:1019-1030 [DOI] [PubMed] [Google Scholar]


