Abstract
BACKGROUND AND PURPOSE: We present a retrospective review of our experience in the endovascular treatment of posterior cerebral artery (PCA) aneurysms. We detail the anatomic location of these aneurysms, the technique of endovascular treatment, morphologic results, and clinical outcome. We also discuss the segmental anatomy of the PCA as it relates to the various neurologic deficits that may result from occlusion of the parent artery.
METHODS: From 1993 to 1998, 20 patients (12 female, eight male; mean age, 44 yrs) harboring a PCA aneurysm were treated via an endovascular approach. One patient had two aneurysms, comprising a total of 21 lesions. Fourteen (66%) of 21 aneurysms were saccular in nature, five (24%) were giant serpentine aneurysms, and two (10%) were posttraumatic. All aneurysms were treated using Guglielmi detachable coils (GDC) either by selective obliteration of the aneurysm sac or by parent artery occlusion.
RESULTS: Fourteen (66%) of the 21 aneurysms were successfully treated with preservation of the parent artery. In the remaining seven (33%), the parent artery was permanently occluded. The overall complication rate in this series was 15%, with a permanent morbidity rate of 10% and a 0% mortality rate.
CONCLUSION: Aneurysms of the PCA are rare compared with other locations in the intracranial circulation. Saccular PCA aneurysms can be treated effectively, by use of GDC, to obliterate the aneurysm yet preserve the parent artery. Fusiform and giant serpentine aneurysms of the PCA can effectively be treated by permanent occlusion of the parent artery; in these cases, thorough knowledge of the PCA segmental anatomy is crucial in order to select the site of occlusion and to avoid major neurologic deficits.
Aneurysms of the posterior cerebral artery (PCA) represent approximately 1% of all intracranial aneurysms (1–4). The surgical approach and dissection of the PCA is technically challenging owing to the complexity of its perforating branches and their intimate relationship with the cranial nerves and upper brain stem. Selective catheterization of the PCA and endovascular treatment of aneurysms arising from its different segments are technically feasible, offering a viable alternative to the surgical approach (5). We discuss the radiographic features, clinical presentation, and possible endovascular treatment of PCA aneurysms with Guglielmi Detachable Coils (GDC). Endovascular treatment with the GDC varies depending on the nature of the aneurysm (ie, saccular, fusiform, or dissecting), and its location along the different anatomic segments of the PCA.
A precise knowledge of the segmental anatomy of the PCA and its branches is essential when the surgical or endovascular approach to an aneurysm is planned, particularly if parent vessel occlusion is contemplated.
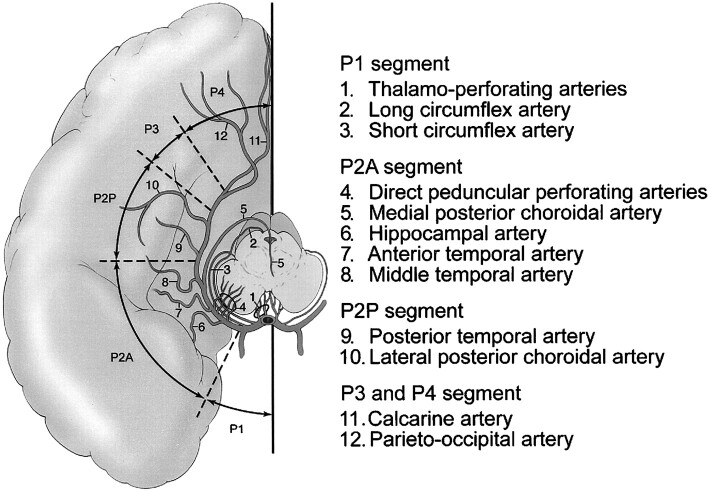
The PCA can be subdivided into four anatomic segments (6–8), as shown in Figure 1. The P1 segment extends from the tip of the basilar artery to the origin of the posterior communicating artery. The P2 segment extends from the posterior communicating artery to the dorsal aspect of the midbrain; this P2 segment can be further subdivided into anterior (P2A) and posterior (P2P) segments. The P3 segment extends from the lateral aspect of the quadrigeminal cistern at the origin of the posterior temporal artery, to the anterior limit of the calcarine fissure. The P4 segment consists of the terminal cortical branches of the PCA, after the takeoff of the parietooccipital and calcarine arteries. Each of these segments gives off groups of branches that supply distinct anatomic territories: brain stem and thalamic branches, ventricular branches, and cortical branches. The four segments of the PCA and their respective branches are detailed in Figure 1.
fig 1.
Anatomic segments of the PCA and their branches.
Composite line tracing of all four anatomic segments of the PCA.
Methods
From 1993 to 1998, we encountered at our institution 20 patients presenting with a PCA aneurysm. One patient had two aneurysms, making a total of 21 aneurysms in this series. The age of our patients ranged from 13 to 78 years with a mean age of 44 years and a slight female preponderance: 12 female/ eight male patients.
The aneurysms were located as follows: seven (33%) at the P1 segment; six (28%) at the junction of the P1 and P2 segments; two (9%) at the P2 segment; three (14%) at the junction of the P2 and P3 segments; two (9%) at the P3 segment; and one (4%) at the junction of the P3 and P4 segments.
We excluded from this series all basilar tip aneurysms that involved the origin of the P1 segment of the PCA. However, in our series, two of the aneurysms arising from the P1 segment were eccentric and extended medially toward the tip of the basilar artery. They both necessitated the use of temporary balloon inflation across the neck during the obliteration of the aneurysm with GDC (“remodeling technique”).
Of the 21 PCA aneurysms, 14 (66%) were saccular, five (24%) were giant serpentine, and two (10%) were posttraumatic. In one of these two patients, there was an additional pseudoaneurysm of the medial tentorial artery. Eight (40%) of the 20 patients presented with subarachnoid hemorrhage (SAH) as a result of rupture of the PCA aneurysm. Six of these eight ruptured aneurysms were of the berry type, one was a giant serpentine aneurysm, and one was a posttraumatic pseudoaneurysm. Four of the 20 patients had multiple intracranial aneurysms involving more than one circulation; two of these four patients with multiple aneurysms presented with SAH from a ruptured aneurysm in another site (anterior circulation aneurysm and posterior communicating aneurysm).
Of the five giant serpentine aneurysms of the PCA, four presented with a constellation of symptoms related primarily to the associated mass effect on the adjacent temporal lobe and optic tract, ie, seizures, memory loss, and incongruous hemianopsia. One 12-year-old boy with a giant serpentine aneurysm presented with intracerebral and subarachnoid hemorrhages (Fig 2).
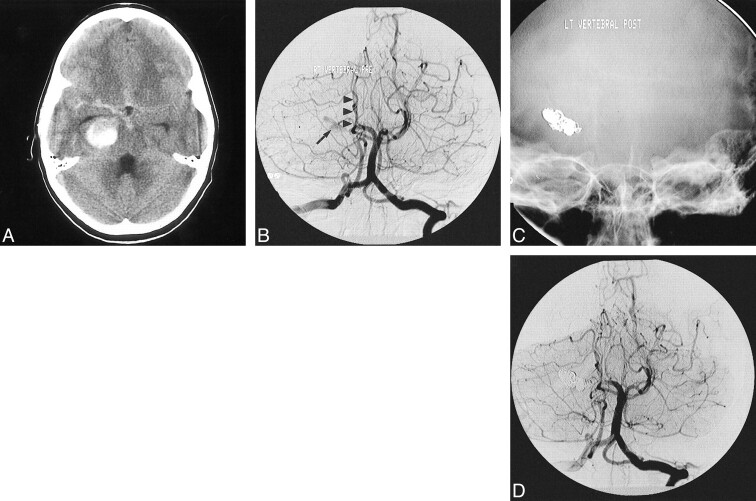
fig 2.
Giant serpentine aneurysm of the P2A segment of the PCA presenting with intracerebral and subarachnoid hemorrhage in a 12-year-old boy.
A, Noncontrast CT scan of the head shows the large partially clotted aneurysm in the perimesencephalic cistern, associated with SAH.
B, Towne's view of the vertebral angiogram shows the residual lumen of the partially clotted aneurysm (arrow), non-filling of the distal branches of the ipsilateral PCA, and avascular mass effect on the brain stem structures (arrowheads).
C, The lumen of the aneurysm and the parent artery were permanently occluded with GDC. The nonsubtracted Towne's view shows the metallic cast of the coils.
D, The corresponding subtracted image of the posttreatment vertebral angiogram shows complete obliteration of the aneurysm.
In all patients, the endovascular treatment was performed under general anesthesia and systemic heparinization. The adequacy of systemic anticoagulation was monitored by frequent measurements of the activated clotting time (ACT). A baseline ACT was obtained prior to the bolus infusion of heparin (50 to 75 IU/kg body weight), and hourly thereafter. The bolus infusion of heparin was followed by a continuous drip (1000 to 1500 IU/hr), with the purpose of doubling the baseline ACT.
We attempted to obliterate saccular aneurysms selectively regardless of their anatomic location along the PCA. Saccular aneurysms with a wide neck were selectively obliterated using the balloon remodeling technique. Partially clotted giant and giant serpentine aneurysms were treated by means of parent artery occlusion, which was not preceded with a test occlusion.
Results
Our results are summarized in the Table. All 20 patients were treated via endovascular approach by use of GDC. Thirteen (61%) of the 21 PCA aneurysms involved the P1 segment or the P1/P2 junction; 10 of these 13 aneurysms were saccular in nature. Two of six aneurysms involving the P1/P2 junction were dissecting in nature and were the result of a major head trauma. In 14 aneurysms treated with GDC, we were able to obliterate the aneurysm successfully while preserving the parent PCA (Fig 3). In the remaining seven, the aneurysm and the parent artery were permanently occluded. For five of these seven patients in whom the parent artery was occluded, the aneurysm was of the giant serpentine type without any identifiable or discrete neck. Two of these seven patients, who were treated with parent artery occlusion, presented with acute SAH early in our experience with endovascular treatment of aneurysms (1993 and 1994); today, however, the parent artery would be preserved in these same patients. One patient with a giant serpentine aneurysm of the P2/P3 segment who was treated with parent artery occlusion developed a contralateral hemiparesis and homonymous hemianopsia (Fig 4). Another patient with a P2-P3 berry aneurysm treated with endovascular occlusion of the proximal P2 segment developed homonymous hemianopsia as a result of the treatment, but without any motor deficit.
TABLE: Summary of 20 patients with 21 PCA aneurysms
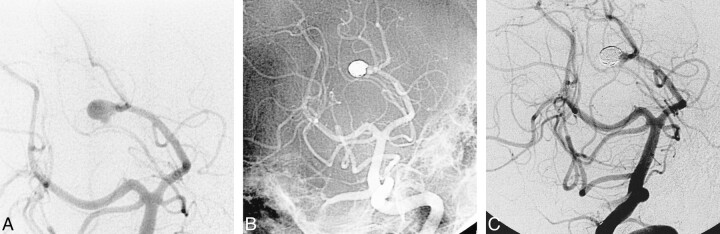
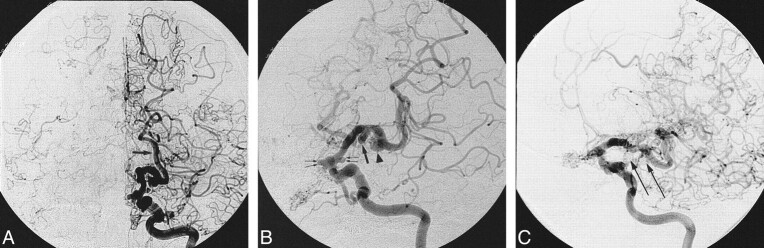
fig 3.
Successful obliteration of a ruptured saccular aneurysm of the PCA with preservation of the parent artery.
A, The oblique subtracted view of a vertebral angiogram shows an irregular bilobed saccular aneurysm arising from the origin of the P3/P4 segments. The patient is a 52-year-old woman presenting with acute SAH.
B, The oblique nonsubtracted view of the posttreatment vertebral angiogram shows the cast of the GDC obliterating the aneurysm with preservation of the parent artery.
C, The oblique subtracted view of the follow-up vertebral angiogram, obtained 2 years after the treatment, shows persistent obliteration of the aneurysm.
fig 4.
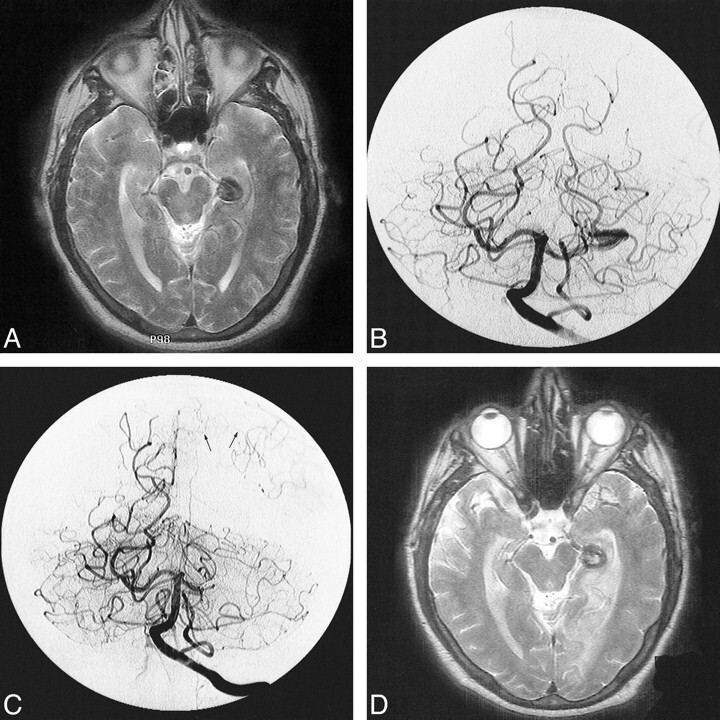
Parent artery occlusion of a giant serpentine aneurysm of the PCA complicated by cerebral infarction in the distal arterial territory.
A, Axial T2-weighted MR image of the head showing a giant serpentine aneurysm of the P2/P3 segments of the left PCA.
B, Towne's view of a left vertebral angiogram showing the serpentine nature of the giant aneurysm, which involves the P2 and P3 segments of the left PCA.
C, Subtracted Towne's view of the posttreatment vertebral angiogram shows obliteration of the aneurysm and no antegrade flow in the distal branches of the left PCA. Note the retrograde flow from the cortical branches of the ipsilateral middle cerebral artery (arrows).
D, Homonymous hemianopsia and contralateral hemiparesis complicated the treatment. The posttreatment axial T2-weighted MR image shows an acute infarction in the distribution of the left PCA territory.
We had one technical complication related to endovascular treatment, with perforation of a P1 segment berry aneurysm in a woman who presented with intracerebral hemorrhage from an ipsilateral occipital arteriovenous malformation (AVM). Despite the perforation, we completely obliterated the aneurysm with GDC and preserved the parent vessel. The patient experienced severe headaches for about 72 hours but did not develop any neurologic deficit related to the technical complication. Her neurologic examination remained normal on follow-up office visits.
In five of seven patients in whom the parent artery was occluded, we were able to demonstrate retrograde collateral filling of the PCA from the middle cerebral circulation. In one of these seven patients in whom the parent artery was occluded at the P3 segment level without any neurologic sequela, there was no demonstrable collateral supply to the distal arterial territory.
Angiographic follow-up is available in 11 of the 14 aneurysms that were obliterated with GDC and where the parent artery was preserved. The follow-up angiogram was performed at an average of 8 months after the endovascular treatment. There was no recanalization of the treated aneurysm on any of the follow-up studies. The 6-month follow-up angiogram is not yet due in two patients; in one patient diagnosed with a Glasgow Outcome Scale (GOS) of 3, the follow-up angiogram was not performed. Of the seven patients who were treated with parent artery occlusion, two underwent follow-up MR imaging and MRA studies showing thrombosis and shrinkage of the aneurysm. The remaining five patients did not undergo follow-up radiographic studies.
The clinical outcome of our patients was assessed upon discharge from the hospital and, where available, at the time of follow-up. The outcome was measured on the GOS; 95% of the patients (19/20) were at a GOS of 1. One patient with a grade IV on the Hunt and Hess scale at the time of treatment was at a GOS of 3 at the time of the clinical follow-up.
In this series, the overall complication rate was 15% (3/20): one procedural aneurysm perforation that did not result in any clinical sequela, and two patients who developed permanent neurologic deficit as a result of permanent artery occlusion. There was no mortality in our series.
Discussion
Aneurysms arising from the PCA have a predilection for the P1 and P2 segments. This is the case in our series (Table) as well as in other previously reported series (2, 3, 9). These aneurysms have some peculiar morphologic features and present with specific clinical findings that distinguish them from aneurysms occurring at other anatomic locations of the intracranial circulation. They frequently affect young patients, with an average age of 38 years (9). This is younger than the average age of 50 to 60 years for aneurysms occurring at other anatomic sites. Also, there is a higher incidence of large and giant aneurysms (almost 23% of PCA aneurysms, versus 3–5% at other anatomic sites) affecting the PCA (1, 10). In his series of PCA aneurysms, Drake (11) reported a 42% incidence of giant aneurysms and Yasargil (7) a 50% incidence of giant aneurysms. In our series, 24% (5/21) of the PCA aneurysms were giant in size, which is similar to the results reported by Pia and Fontana (3).
The most common clinical presentation of PCA aneurysms described in the literature is the SAH (80%) (2, 3, 9). In our series, half of the patients presented with SAH (10/20 patients, 50%) and in two of these patients, the SAH was related to a rupture of a remote aneurysm. The majority of our patients presenting with SAH had a berry aneurysm (8/10, berry aneurysms; 1/10, giant serpentine aneurysm; 1/10, dissecting aneurysm). In five of our patients, the PCA aneurysm was an incidental finding (4/5, berry aneurysm; 1/5, giant serpentine aneurysm), without any associated neurologic deficit.
In general, giant serpentine aneurysms have a predilection for the posterior circulation and affect the younger population (2, 3, 9, 13). They can present with signs of mass effect on the surrounding brain parenchyma, resulting in seizures or other neurologic deficit (14). One of our patients with a giant serpentine aneurysm of the P3 segment presented with memory loss; another patient with a giant serpentine aneurysm of the P2-P3 junction presented with sleepiness and memory loss as a result of compression of the brain stem and hippocampus.
Pia and Fontana (3) report a 27% incidence of visual disturbances in patients harboring PCA aneurysms, with a prevalence of oculomotor palsy and hemianopsia. In our own series, five patients (25%) had visual or oculomotor disturbances: one patient with a P1 segment giant serpentine aneurysm compressing the optic chiasm had a bitemporal hemianopsia, and another patient with a P1 segment berry aneurysm and a coexisting ipsilateral occipital AVM had a homonymous hemianopsia. Three other patients with a P1-P2 junction aneurysm, two of whom with a posttraumatic pseudoaneurysm, presented with a CN III palsy.
In our patient population of 21 PCA aneurysms, we observed a relatively high incidence of coexisting vascular dysplasias. Nine (43%) of the 21 PCA aneurysms were associated with other vascular lesions: three patients had an occipital AVM supplied by the ipsilateral PCA (Fig 5), four patients had multiple intracranial aneurysms, one patient had Moya-Moya disease (Fig 6), and one patient had a posttraumatic dissecting aneurysm of the posterior tentorial artery. All of the PCA aneurysms associated with other vascular dysplasias were of the berry type, whereas none of the five giant serpentine aneurysms was associated with other vascular lesions. Our data therefore suggest that berry aneurysms of the PCA, although rare, are often the result of increased flow in the posterior circulation. The underlying hemodynamic conditions predisposing to increased flow include: AVM, Moya-Moya disease resulting in exuberant collateral circulation, and collateral supply to the intracranial carotid circulation through the PCA after iatrogenic ligation of the proximal carotid artery. Only two P1 segment berry aneurysms were isolated lesions. The other PCA berry aneurysms, which were not the result of high-flow conditions, were indeed associated with other intracranial aneurysms and other conditions such as polycystic kidney disease.
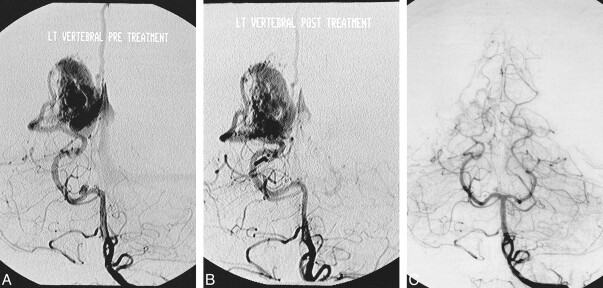
fig 5.
P1 segment aneurysm associated with a distal cortical AVM.
A, Pretreatment Towne's view of a vertebral angiogram showing a P1 segment aneurysm associated with a temporooccipital AVM. Note the poor ratio of the fundus of the aneurysm to the size of its neck.
B, Same view of the vertebral angiogram after the endovascular treatment of the aneurysm with GDC
C, Follow-up vertebral angiogram obtained 42 months after the initial treatment showing complete obliteration of the AVM and questionable minimal residual filling of the aneurysm.
fig 6.
Multiple PCA associated with Moya-Moya disease, successfully treated with GDC.
A, Frontal view of left internal carotid angiogram showing occlusion of the anterior and a middle cerebral arteries and Moya-Moya collateral circulation. There is increased flow in a dysplastic and enlarged ipsilateral PCA (arrow).
B, Oblique view of the internal carotid angiogram showing an aneurysm originating from the P1/P2 junction of the left posterior cerebral artery (arrow) and a second aneurysm arising from the P2P segment (arrowhead). Note the presence of at least two flow-related aneurysms arising from the supraclinoid segment of the internal carotid artery (double arrows).
C, Oblique view of the internal carotid angiogram, obtained 9 months after treatment, shows persistent obliteration of both PCA aneurysms (arrows).
In those patients who present with an AVM supplied by the PCA and have an associated berry aneurysm arising from the parent vessel, we prefer to obliterate the aneurysm first by endovascular means prior to treating the AVM. This is particularly true when the aneurysm is irregular and located close to the tip of the basilar artery (P1 segment). Although some of the flow-related aneurysms, particularly the ones that are sessile and peripherally located, may involute or decrease in size after treatment of the AVM, we have encountered some in our practice that did not change in size and some that indeed presented with SAH. Once the aneurysm is obliterated, the AVM is then treated with whatever method necessary, including embolization with liquid adhesive.
In our opinion, the treatment of choice of berry aneurysms of the PCA is selective endovascular obliteration with GDC, with preservation of the parent artery. The endovascular obliteration with GDC of small aneurysms with a small neck is safe and effective and, in our series, associated with good long-term anatomic results. As with any other arterial location, large sessile aneurysms with wide necks are generally not well suited for endovascular obliteration with GDC and are better treated with surgical clipping. Occasionally, wide-neck aneurysms of the PCA can be obliterated with GDC by use of a balloon-assisted technique to avoid poor packing of the coils and/or encroachment on the parent artery. When necessary (2/21 aneurysms), we performed this technique safely with excellent anatomic results. We do not believe, however, that aggressive and risky endovascular treatment should be performed at the cost of increasing the morbidity of the technique or in lieu of straightforward surgical clipping of aneurysms.
Preservation of antegrade flow in the PCA must naturally be one of the prime objectives in the treatment of aneurysms arising from this vessel. However, in the case of fusiform or giant serpentine aneurysm, parent artery occlusion becomes inevitable in order to obliterate the aneurysm. In these cases, thorough anatomic knowledge of the various segments (Fig 1) of the PCA and their functional territories becomes crucial in order to predict and/or avoid the neurologic deficit that may occur as a result of their purposeful occlusion.
In our patients in whom the treatment of the PCA aneurysm required permanent artery occlusion, there was a relatively low incidence of visual field defect (1/7 or 14.2%). The low incidence of visual field defect complicating parent artery occlusion is related to the rich anastomotic collaterals that exist between the territory of the PCA and that of other arteries, which include:
1) Collateral circulation between the lateral posterior choroidal (branch of the P2P segment) and the anterior choroidal arteries.
2) Collateral circulation between the long circumflex arteries (branches of the P1 segment) and the superior cerebellar artery territory at the level of the quadrigeminal plate.
3) Collateral circulation between the splenial artery (branch of the P3-P4 segments) and the posterior pericallosal artery (branch of the anterior cerebral artery).
4) Collateral circulation between the inferior temporal branches of the PCA and the superior temporal branches of the middle cerebral artery.
Unfortunately, these anastomotic collateral channels are not easily recognizable on routine subtraction angiography and cannot be adequately evaluated prior to a planned artery occlusion. Therefore, and in our experience, it is impossible to be certain whether or not a parent artery occlusion will be tolerated. In general, however, we avoid parent artery occlusion at the P1 or P2 segments given the rich vascular supply to the brain stem that originates from these anatomic locations and the possible neurologic deficits that may occur as a result of such treatment.
One of our patients who presented with a homonymous hemianopsia due to an infarct of the calcarine cortex had a posttraumatic dissecting aneurysm of the P1/P2 junction. These aneurysms that rarely occur in the posterior circulation typically present with severe neurologic deficits due to cerebral infarction and hemorrhagic complications (3, 15). The two dissecting aneurysms of the PCA encountered in our series were posttraumatic in nature and were the result of a major motor vehicle accident, resulting in a severe blow to the head. They both occurred at the junction of the P1 and P2 segments of the PCA with the posterior communicating artery. Both of these patients presented with a CN III palsy due to the shearing injury of the cranial nerve. We postulate that the significant shear force applied to the posterior communicating artery was transmitted to the PCA and resulted in a focal laceration. As the arterial laceration healed, it became walled off and formed a false posttraumatic aneurysm. The same shearing trauma also explains the injury to the third nerve. Another possible mechanism in the pathogenesis of these aneurysms could be the mere stretching of the PCA against the fixed and rather rigid edge of the tentorium. Of particular interest is the therapeutic management of such aneurysms, which has not been widely reported, specifically with regard to the appropriateness of immediate treatment (10, 16). In general, dissecting aneurysms of the posterior circulation have a very high mortality rate. However, in the case of exclusive involvement of the PCA, the clinical outcome can be more favorable with reported cases of spontaneous resolution (16–18).
We treated the two dissecting aneurysms of the PCA encountered in our series with endovascular treatment. They were both obliterated with GDC while preserving the antegrade flow in the PCA. Both patients had a follow-up angiogram showing the persistent obliteration of the aneurysm and patency of the parent vessel.
Conclusion
Aneurysms of the PCA are rare but present distinct anatomic and pathophysiological characteristics. Their location along the four anatomic segments of the PCA affects their treatment and corresponding long-term clinical outcome.
In our experience, endovascular treatment of these aneurysms is relatively safe and effective with low mortality and morbidity.
Footnotes
Address reprint requests to M. Mawad, MD, 6565 Fannin, D 281, Houston Texas 77030.
References
- 1.Drake CG. Giant posterior cerebral aneurysms: 66 patients. In: Drake CG, Peerless SJ, Hruesniemi JA, eds. Surgery of Vertebrobasilar Aneurysms: London, Ontario, Experience on 1767 Patients. New York: Springer Verlag 1996;;230-248
- 2.Gerber CJ, Neil-Dwyer G. A review of the management of 15 cases of aneurysms of the posterior cerebral artery. Br J Neurosurg 1992;;6:521-527 [DOI] [PubMed] [Google Scholar]
- 3.Pia HW, Fontana H. Aneurysms of the posterior cerebral artery. Locations and clinical pictures. Acta Neurochir (Wien) 1977;;38:13-35 [DOI] [PubMed] [Google Scholar]
- 4.Sakata S, Fujii K, Matsushima T, et al. Aneurysms of the posterior cerebral artery: report of eleven cases- surgical approaches and procedures. Neurosurgery 1993;;32:163-167 [DOI] [PubMed] [Google Scholar]
- 5.Guglielmi G, Vinuela F, Duckwiler G, et al. Endovascular treatment of the posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg 1992;;77:515-524 [DOI] [PubMed] [Google Scholar]
- 6.Seoane ER, Tedeschi H, de Oliveira E, Siqueira MG, Calderon GA, Rhoton AL Jr. Management strategies of posterior cerebral artery aneurysms: a proposed new surgical classification. Acta Neurochir (Wein) 1997;;139:325-331 [DOI] [PubMed] [Google Scholar]
- 7.Yasargil MG. Microneurosurgery. Vol 2. Stuttgard: George thieme Verlag 1984;;260-269
- 8.Zeal AA, Rhoton AL. Microsurgical anatomy of the posterior cerebral artery. J Neurosurg 1978;;48:534-559 [DOI] [PubMed] [Google Scholar]
- 9.Ferrante L, Acqui M, Trillo’ G, Lunardi P, Fortuna A. Aneurysms of the posterior cerebral artery: do they present specific characteristics? Acta Neurochir (Wien) 1996;;138:840-852 [DOI] [PubMed] [Google Scholar]
- 10.Lempert TE, Halbach VV, Higashida RT, et al. Endovascular treatment of pseudoaneurysms with electrolitically detachable coils. AJNR Am J Neuroradiol 1998;;19:907-911 [PMC free article] [PubMed] [Google Scholar]
- 11.Drake CG. Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg 1979;;26:12-95 [DOI] [PubMed] [Google Scholar]
- 12.Drake CG, Amaker AL. Aneurysms of the posterior cerebral artery. J Neurosurg 1969;;30:468-474 [DOI] [PubMed] [Google Scholar]
- 13.Fukamachi A, Hirato M, Wakao T, Kawafuchi J. Giant serpentine aneurysm of the posterior cerebral artery. Neurosurgery 1982;;11:271-276 [DOI] [PubMed] [Google Scholar]
- 14.Yacubian EM, Rosemberg S, da Silva HC, Jorge CL, de Oliveira E, de Assis LM. Intractable complex partial seizures associated with posterior cerebral artery giant aneurysm: a case report. Epilepsia 1994;;36:1317-1320 [DOI] [PubMed] [Google Scholar]
- 15.Deruty R, Mottolese C, Bascoulergue Y, Pialat J. Spontaneous progressive thrombosis and surgical resection of a giant aneurysm of the posterior cerebral artery in an 18-year-old. Neurol Res 1987;;9:169-176 [DOI] [PubMed] [Google Scholar]
- 16.Nanda A, Drury BT. Posterior circulation aneurysm in a child: clipping of one leads to spontaneous thrombosis of the other. Pediatr Neurosurg 1997;;26:41-47 [DOI] [PubMed] [Google Scholar]
- 17.Maillo A, Diaz P, Morales F. Dissecting aneurysm of the posterior cerebral artery: spontaneous resolution. Neurosurgery 1991;;29:291-293 [DOI] [PubMed] [Google Scholar]
- 18.Pozzati E, Padovani R, Fabrizi A, Sabattini L, Gaist G. Benign arterial dissections of the posterior circulation. J Neurosurg 1991;;75:69-72 [DOI] [PubMed] [Google Scholar]