Abstract
BACKGROUND AND PURPOSE: Few investigators have analyzed the MR imaging patterns of fetal gyration. Our purpose was to establish, with a large prospective series, the normal sulcation landmarks according to gestational age by using in utero MR imaging and to correlate our findings with established neuroanatomic timetables.
METHODS: A standardized fetal cerebral MR examination was performed in 173 normal fetuses at 22 to 38 weeks' gestation. Eight T1- and T2-weighted coronal, axial, and sagittal slices were obtained for each fetus and systematically analyzed. The sequential development of the different fissures and sulci of the cerebral cortex with respect to gestational age were tabulated.
RESULTS: Sulcation of the medial, lateral, and inferior surfaces of the brain was depicted, and a timetable for the MR depiction of the primary and secondary sulci was established for the 22- to 38-week gestational period. This timetable was in good agreement with the neuroanatomic standards of reference, with a mean lag time of 1 week.
CONCLUSION: This analysis of fetal brain sulcation in a large series of fetuses contributes to a better understanding of the maturation of the fetal cortex on MR imaging studies. It furthermore provides a standard of reference that can be used to assess the normality of fetal sulcation and to diagnose gyrational abnormalities with prenatal MR imaging.
Gyration is a phenomenon occurring late during fetal development and can be observed by the second month of intrauterine life. It goes on to the end of the pregnancy and even later after birth. The primary sulci appear as shallow grooves on the surface of the brain that become progressively more deeply infolded and that develop side branches, designated secondary sulci. Gyration proceeds with the formation of other side branches of the secondary sulci, referred to as tertiary sulci (1, 2). The timing of the appearance of these different types of sulci is so precise that neuropathologists consider gyration to be a reliable estimate of gestational age and consequently a good marker of fetal brain maturation.
Fetal gyration has been studied neuropathologically (3–5) and sonographically in preterm neonates (6, 7) and in fetuses with (8) or without (9) a transvaginal probe. However, sonography provides an inconsistent and incomplete depiction of the different sulci. The patterns of fetal sulcation have also been described on MR images in vitro (10) and in preterm neonates in vivo (2), but few investigators have reported the MR appearance of in vivo antenatal gyration (11). Moreover, the reports that exist lack standardized protocols dedicated to the examination of the normal fetal brain. Indeed, obvious ethical reasons make it impossible to perform MR imaging on “normal” fetuses.
Our purpose was to describe the patterns of normal fetal sulcation on cerebral MR examinations in a large series of fetuses that were studied prospectively using a standardized protocol. This study thus provides MR standards of reference for the average time of development of the fissures and sulci.
Methods
Among the 500 cerebral fetal MR examinations performed in our institution, we selected 173 studies of fetuses with a gestational age of 22 to 38 weeks. Gestational age was determined by a sonographic study performed at 12 weeks' gestation. The distribution of the fetuses according to gestational age is given in Figure 1. Fetuses of 22 to 23 weeks' gestation, 24 to 25 weeks' gestation, and 37 to 38 weeks' gestation were grouped together because they were insufficient in number to warrant separate categorization; moreover, we could not observe marked changes in gyrational patterns within a 2-week time frame. All fetuses showed normal findings on sonographic brain examinations. MR imaging was performed within standardized exploration protocols for fetal indications of anomalies commonly associated with cleft palate, cardiac rhabdomyoma, familial history of gyrational abnormalities, polyhydramnios, poor movement activity, and bilateral club feet. All the fetuses had normal cerebral MR findings. Subsequent examinations showed their brains to be normal either at neuropathologic examination (in case of an interrupted pregnancy for extracerebral reasons) or at sonography and/or MR imaging performed postnatally. Clinical neurologic follow-up at 1 to 3 years (average, 2 years) was normal as well. Twin pregnancies were excluded from this study. Sex and side of the brain imaged were not taken into account.
fig 1.
Distribution of fetuses by gestational age (GA)
The study was performed within a series of fetal MR examinations acquired prospectively using standardized dedicated protocols (slices always orthogonal to the fetal brain) and a 0.5-T superconducting magnet. Sedation was obtained by oral administration of flunitrazepam (1 mg). MR images were acquired by using two types of sequences (surface or body coil): gradient-echo T1-weighted sequences were performed with parameters of 300/15/4 (TR/TE/excitations), a flip angle of 75°, a slice thickness 5 mm, a field of view (FOV) of 32 × 32 cm, and a matrix of 200 × 200. Steady-state free procession gradient-echo sequences were performed with a heavily T2-weighted sequence (E-short) and parameters of 20/9.2/12, a flip angle of 70°, a slice thickness of 5 mm, an FOV of 32 × 32 cm, and a matrix of 200 × 200.
To study gyrational patterns, eight slices were systematically analyzed for each fetus, and studies were included only if these eight slices were available:
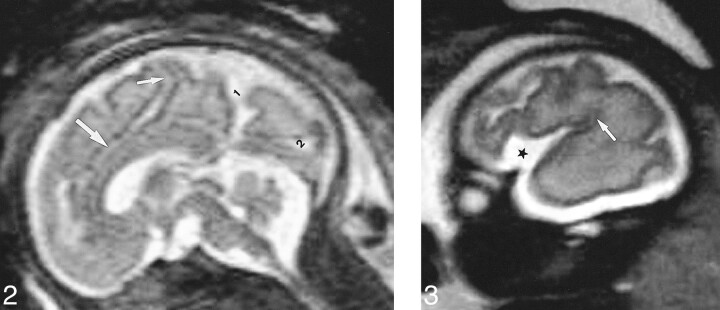
Two sagittal slices—one midline T2-weighted slice (no. 1) to study the sulcal anatomy of the medial cerebral surface (Fig 2) and one lateral T1- or T2-weighted slice (no. 2) at the level of the sylvian fissure to analyze its operculation (Fig 3).
fig 2.
Slice no. 1. T2-weighted midline sagittal image (20/9.2/12) (31 weeks' gestation). 1, parietooccipital fissure; 2, calcarine fissure; small arrow, marginal sulcus; large arrow, cingular sulcus.fig 3. Slice no. 2. T2-weighted parasagittal image (20/9.2/12) at the level of the sylvian fissure (28 weeks' gestation). Opercularization of the insula is completed posteriorly (arrow). Anteriorly, the insula (star) is wide open
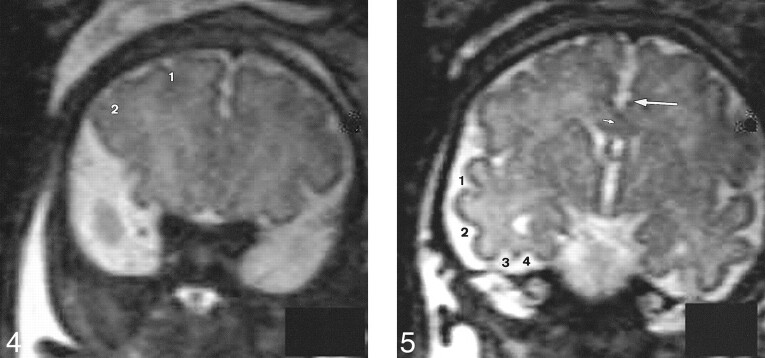
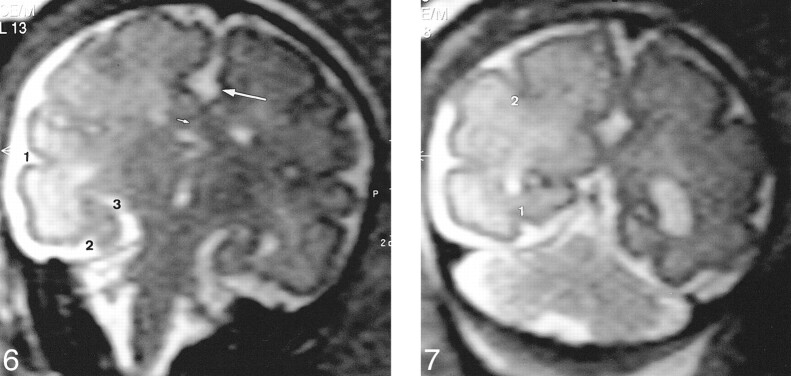
Four T2-weighted coronal slices parallel to the brain stem—at the anterior part of the frontal lobes (no. 3) (Fig 4), at the level of the third ventricle (no. 4) (Fig 5), at the level of the temporal horns (no. 5) (Fig 6), and at the level of the ventricular atria (no. 6) (Fig 7). These slices were dedicated to the sulcal anatomy of the medial, lateral, and ventral cerebral surfaces.
fig 4.
Slice no. 3. T2-weighted anterior coronal image (20/9.2/12) at the anterior part of the frontal lobes (35 weeks' gestation). 1, superior frontal sulcus; 2, inferior frontal sulcus.fig 5. Slice no. 4. T2-weighted coronal image (20/9.2/12) at the level of the third ventricle (35 weeks' gestation). Large arrow, cingulate sulcus; small arrow, callosal sulcus (barely visible); 1, superior temporal sulcus (anterior part); 2, inferior temporal sulcus; 3, external occipital temporal sulcus; 4, collateral sulcus.
fig 6.
Slice no. 5. T2-weighted coronal image (20/9.2/12) at the level of the temporal horns (33 weeks' gestation). Large arrow, cingulate sulcus; small arrow, callosal sulcus (barely visible); 1, superior temporal sulcus (posterior part); 2, collateral sulcus; 3, hippocampal fissure.fig 7. Slice no. 6. T2-weighted coronal image (20/9.2/12) at the level of the ventricular atria (33 weeks' gestation). 1, collateral sulcus; 2, intraparietal sulcus
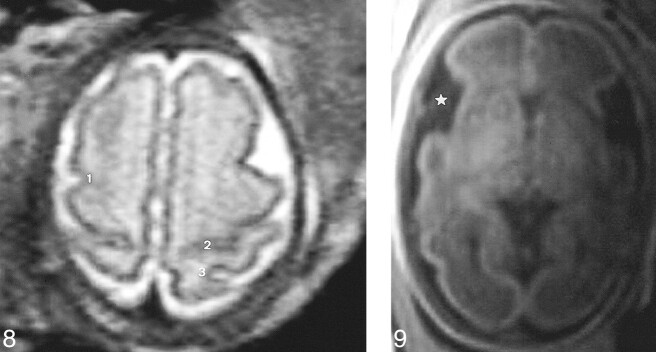
One T1- or T2-weighted transverse slice perpendicular to the brain stem at the level of the vertex (no. 7) to study the anatomy of the precentral, postcentral, and central sulci (Fig 8).
fig 8.
Slice no. 7. T2-weighted transverse image (20/9.2/12) at the level of the vertex (31 weeks' gestation). 1, precentral sulcus; 2, central sulcus (almost abutting the hemispheric fissure); 3, postcentral sulcus.fig 9. Slice no. 8. T1-weighted transverse image (300/15/4) at the level of the third ventricle (29 weeks' gestation). The insula (star) is wide open
One transverse T1-weighted slice at the level of the third ventricle (no. 8) (Fig 9) to study the insula.
The secondary cingular sulci and the occipital sulci were evaluated on the midline sagittal slice and the insular sulci on the lateral and coronal slices. A clear indentation at the surface of the brain was considered the earliest indication of a sulcus. Moreover, a sulcus was considered to be present if it was observed in more than 75% of cases, detectable if observed in 25% to 75% of cases, and absent if observed in less than 25% of cases. The results were compared with known neuroanatomic timetables (3).
Results
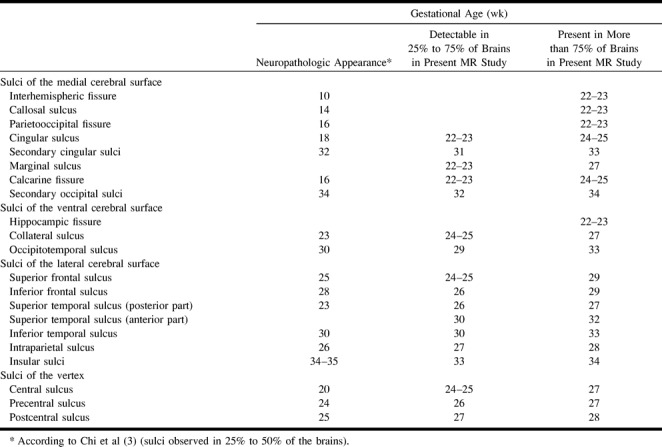
The periods of detectability and the presence of the various sulci and fissures are summarized in the Table. There was a 2-week mean lag time between the detectability of a sulcus and its presence in more than 75% of the cases.
Chronology of sulcation in our MR imaging series and in neuropathologic studies
The callosal sulcus was often seen better on the coronal slice than on the midline sagittal slice because of a partial volume effect. By 22 weeks' gestation, the interhemispheric fissure, the callosal sulcus, the parietooccipital fissure, and the hippocampic fissures were always present. Initially, the superior temporal sulcus was detected posteriorly at 26 weeks' gestation and extended anteriorly so that its anterior portion was detected only at 30 weeks' gestation. The central sulcus emerges laterally and was detected at 24 to 25 weeks' gestation; it then extends medially, and finally abuts the interhemispheric fissure by 30 weeks' gestation.
By 33 weeks' gestation, all the primary sulci were present. At 31 weeks' gestation, the cingular sulcus becomes tortuous because of the development of secondary sulci. The same phenomenon was observed in the calcarine fissure with the development of secondary occipital sulci. At 34 weeks' gestation, the insular, cingular, and occipital secondary sulci were present.
The times of appearance of the different sulci and fissures at neuropathologic examination, according to the work of Chi et al (3), are summarized in the first column of the Table. These investigators indicated the gestational age at which 25% to 50% of brains showed the sulcal structures, and their findings are comparable to our times of detectability in 25% to 75% of brains.
The time of appearance of the sulci is delayed by a mean lag time of 1 week on MR images as compared with neuropathologic findings. The greatest lags are observed for the cingular sulcus, the calcarine fissure, the superior temporal sulcus, and the central sulcus.
The sylvian fissure was observed in all fetuses at 22 to 23 weeks' gestation. It appears as a shallow depression at the surface of the brain, and the insula is wide open at this time. The temporal lobe folds over to engulf the insula, and this progressive closure of the insula begins posteriorly and extends anteriorly. This phenomenon was observed on slice nos. 2, 4, and 8 (Figs 3, 5, and 9). By 33 weeks' gestation, insular sulci are recognizable and the surface of the sylvian fissure becomes more and more indented.
Discussion
Cortical sulcation is considered to be a good marker of fetal brain maturation by neuropathologists (3–5); however, even among these researchers, there are discrepancies concerning the time of appearance of some sulci: according to Larroche (5), the superior temporal sulcus is a reliable morphologic criterion of gestational age and appears at 28 weeks' gestation, whereas Chi et al (3) observed it by 24 to 26 weeks' gestation. Moreover, the central sulcus is detectable at 20 weeks' gestation according to Chi et al (3) and Larroche (5) and at 24 weeks' gestation according to Dorovini-Zis and Dolman (4).
These discrepancies may be attributed to several factors: 1) gestational age, which was not based on the same criteria for all the authors—last menstrual period for some (3, 5) (which is known to be unreliable), head circumference, crown-to-heel length and general pathologic findings for others (4); 2) size of the cohort, which varied from 30 (5) to 80 (4) to 207 (3) fetuses; 3) neuropathologic technique used, including gross inspection of the brain, photographs of the fixed brains, serial sections of the brain with thicknesses varying from 15 to 30 μ; and 4) brain side and inclusion of twin fetuses, which may influence sulcation (in fact, a delay of 2 or 3 weeks has been reported in the development of sulcation patterns in the brains of twins vs nontwins aged 19 to 32 weeks' gestation) (3). These discrepancies emphasize the difficulty of establishing reliable patterns of sulcation with a high degree of precision.
As mentioned earlier, patterns of sulcation have also been determined with sonography and MR imaging. Our aim was not to compare MR with sonography. Most sonographic studies (6, 7, 8) use a transfontanellar approach and therefore obtain radiated slices in a fan-shaped fashion from the anterior fontanelle. Therefore, one cannot compare these slices with anatomic or MR findings. Sonography is usually used to study essentially the medial surface of the brain and the sylvian fissure. The convexity and the frontal lobe are difficult to image. Furthermore, many features inherent to sonography limit the depiction of sulcation: the inability to achieve a transfontanellar approach because of the position of the fetal head, interference of the sonographic beam, and penetration by fatty maternal structures. MR imaging offers several advantages over sonography, such as multiplanar capability independent of the position of the fetal head and better contrast between the brain and the pericerebral fluid. However, one must keep in mind that at the end of the pregnancy (after 34–35 weeks' gestation) the subarachnoid space is less wide and does not allow good delineation of the sulci (12).
Few investigators have studied the MR imaging pattern of fetal gyration, which is understandable, given the paucity of large series of “normal” fetal cerebral MR examinations. In a recent article assessing the cortical maturation of the fetal brain on MR images, 28 of the 53 examinations were performed for maternal indications, and imaging planes were therefore often nonorthogonal to the fetal brain (11). According to these authors, this was a bias that could explain a delay in the appearance of sulci and fissures, and they underlined the need for further studies to substantiate their findings. In studies of the maturation of the fetal cortex, gestational age is frequently based on the mother's last menstrual period and not on early sonographic features. Furthermore, among the 53 fetuses analyzed by Levine and Barnes (11), six twin pregnancies were included, which may constitute another bias in the evaluation of cortical maturation.
In our series, we consistently used sections that were orthogonal to the fetal brain so that we could reliably compare the findings with neuroanatomic data. Our results lag behind those of neuropathologists (3–5) by an average of 1 week. This discrepancy may be explained by the MR slice thickness used, which was much greater than that used by the neuropathologists, and by the lack of spatial resolution in MR imaging. Conversely, some sulci were detected on MR images before the time predicted by the anatomic specimens: by 1 week for the occipitotemporal and secondary cingular sulci and by 2 weeks for the inferior frontal and secondary occipital sulci. One factor that may account for this delay is that we did not take into account the side of the brain imaged, whereas, according to Chi et al (3), there is an obvious left-right asymmetry in the development of the secondary sulci. It is not clear whether its timetable indicates the right or left hemisphere. The fact that we did not account for the sex of the fetuses is probably not relevant in explaining the delay between the MR and neuropathologic timetables. Indeed, according to Chi et al (3), there is no difference between male and female brain sulcation.
Our results show that, on MR images, the best correlation between sulcal development and gestational age may be obtained from the sulci detectable after 28 weeks' gestation. At 34 weeks' gestation, all the primary and most of the secondary sulci are already present, and, as mentioned earlier, after this time, the sulci are usually not well delineated. For those reasons, we suggest that the best period for performing MR imaging to study gyration of the fetal brain is between 28 and 34 weeks' gestation. Moreover, when analyzing gyration of the fetal brain, we would advise referring to the last column in the Table, referring to the presence of the different sulci. Although the use of detectability as an index of fetal brain maturation is more sensitive, it is not specific enough, and it does not seem useful to determine delayed gyration on the basis of detectability alone.
Naidich et al (1) stressed the role of the orbital sulci in the assessment of brain maturation in late gestation. The olfactory sulci become well defined by 25 weeks' gestation. By 28 to 31 weeks, the orbital sulci develop progressively, and after 36 to 39 weeks, the secondary sulci define the anterior and posterior orbital gyri (1, 3). In our study, we did not analyze systematically the ventral surface of the frontal lobes and therefore we cannot determine the feasibility of such an evaluation. We tried to mark these sulci in a few cases, and we can underline two difficulties: the lack of spatial resolution (which can certainly be improved by using a section thickness of 3 mm instead of 5 mm) and the fact that the analysis is relevant in late pregnancy, when the thinness of the pericerebral fluid makes it difficult to delineate the different sulci. A good depiction of the olfactory sulci could allow the diagnosis of arrhinencephaly.
Conclusion
The MR findings in this large series of normal fetal brains contribute to a better understanding of cortical maturation in fetuses. Fetal sulcation follows a predictable timetable, which, on MR images, slightly lags behind the neuroanatomic one. Our study was based on the analysis of 5-mm-thick MR slices. In all probability, faster imaging techniques—with fewer artifacts, thinner slices, and more adaptable coils—should decrease this delay between neuroanatomic and MR imaging findings. As for myelination after birth, individual variations in gyrational chronology have to be taken into account in any analysis of fetal brain studies. This series provides standards of reference that can be used to assess normality of fetal sulcation and to recognize gyrational abnormalities on prenatal MR images.
Acknowledgments
We are grateful to Christine Beslier for preparation of the manuscript and to Frédéric Virvaire for informatic assistance.
Footnotes
Address reprint requests to Catherine Garel, MD, Department of Pediatric Radiology, Robert Debré Hospital, 48, Bd Serurier, 75935 Paris Cedex 19, France.
References
- 1.Naidich TP, Grant JL, Altman N, et al. The developing cerebral surface. Neuroimaging Clin N Am 1994;4:201-240 [PubMed] [Google Scholar]
- 2.Van der Knaap MS, Van Wezel-Meijler G, Barth PG, Barkhof F, Ader HJ, Valk J. Normal gyration and sulcation in preterm and term neonates: appearance on MR images. Radiology 1996;200:389-396 [DOI] [PubMed] [Google Scholar]
- 3.Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol 1977;1:86-93 [DOI] [PubMed] [Google Scholar]
- 4.Dorovini-Zis K, Dolman CL. Gestational development of brain. Arch Pathol Lab Med 1977;101:192-195 [PubMed] [Google Scholar]
- 5.Larroche JC. Critères morphologiques du développement du système nerveux central du foetus humain. J Neuroradiol 1981;8:93-108 [PubMed] [Google Scholar]
- 6.Huang CC. Sonographic cerebral sulcal development in premature newborns. Brain Dev 1991;13:27-31 [DOI] [PubMed] [Google Scholar]
- 7.Murphy NP, Rennie J, Cooke RW. Cranial ultrasound assessment of gestational age in low birthweight infants. Arch Dis Child 1989;64:569-572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteagudo A, Timor-Tritsch IE. Development of fetal gyri, sulci and fissures: a transvaginal sonography study. Ultrasound Obstet Gynecol 1997;9:222-228 [DOI] [PubMed] [Google Scholar]
- 9.Droulle P, Gaillet J, Schweitzer M. Maturation of the fetal brain echoanatomy: normal development, limits and value of pathology [in French]. J Gynecol Obstet Biol Reprod 1984;13:228-236 [PubMed] [Google Scholar]
- 10.Hansen PE, Ballesteros MC, Soila K, Garcia L, Howard JM. MR imaging of the developing human brain, 1: prenatal development. Radiographics 1993;13:21-36 [DOI] [PubMed] [Google Scholar]
- 11.Levine D, Barnes PD. Cortical maturation in normal and abnormal fetuses as assessed with prenatal MR imaging. Radiology 1999;210:751-758 [DOI] [PubMed] [Google Scholar]
- 12.Garel C, Brisse H, Sebag G, Elmaleh M, Oury JF, Hassan H. Magnetic resonance imaging of the fetus. Pediatr Radiol 1998;28:201-211 [DOI] [PubMed] [Google Scholar]