Abstract
Narghile use has regained popularity throughout the world. Public opinion misjudges its chronic harmful effects on health, especially on the cardiovascular system. This systematic review aimed to evaluate the chronic effects of narghile use on cardiovascular response during exercise. It followed the preferred reporting items for systematic reviews guidelines. Original articles from PubMed and Scopus published until January 31, 2020, written in English, and tackling the chronic effects of narghile use on human cardiovascular response during exercise were considered. Five studies met the inclusion criteria. Only males were included in these studies. They were published between 2014 and 2017 by teams from Tunisia (n = 4) and Jordan (n = 1). One study applied the 6-min walk test, and four studies opted for the cardiopulmonary exercise test. Narghile use was associated with reduced submaximal (e.g., lower 6-min walk distance) and maximal aerobic capacities (e.g., lower maximal oxygen uptake) with abnormal cardiovascular status at rest (e.g., increase in heart rate and blood pressures), at the end of the exercise (e.g., lower heart rate, tendency to chronotropic insufficiency) and during the recovery period (e.g., lower recovery index). To conclude, chronic narghile use has negative effects on cardiovascular response to exercise with reduced submaximal and maximal exercise capacities.
Keywords: Circulatory system, exercise tests, hookah, hubble bubble, waterpipe, physical activity, walk test
Cigarette smoking is a public health problem, associated with an increased risk of cardiovascular diseases (CVDs), chronic obstructive pulmonary diseases, cancers, and mortality (Qasim et al., 2019; Taghizadeh et al., 2016). Public health policies have been successfully implemented to reduce cigarette smoking in many countries (WHO, 2015). Faced with the cigarette-oriented control policies (WHO, 2015), narghile use—another old form of tobacco—has been flourishing and regaining popularity worldwide (Pratiti & Mukherjee, 2019; WHO, 2015), thus becoming an emerging and alarming public health threat (Ben Saad, 2009, 2010; Bou Fakhreddine et al., 2014; Chaouachi, 2009; El-Zaatari et al., 2015; Pratiti & Mukherjee, 2019; Waziry et al., 2017; WHO, 2015). Prevalence of narghile use has increased, and it is the highest in the Eastern Mediterranean Region (WHO, 2015). Moreover, in some countries, prevalence of narghile use has increased, becoming higher than cigarette smoking in certain subgroups (WHO, 2015). Public opinion misjudges the harmful effects of narghile use (Pratiti & Mukherjee, 2019; WHO, 2015). The latter is considered “safer” than cigarette smoking by the general public (Pratiti & Mukherjee, 2019; WHO, 2015), although this smoking mode has been reported to be associated with several adverse health effects, including numerous systems (e.g., cardiorespiratory, hematological, reproductive, and metabolic systems) (Ben Saad, 2010; Ben Saad et al., 2011; Bou Fakhreddine et al., 2014; El-Zaatari et al., 2015; Waziry et al., 2017). Among the deleterious effects of narghile use, CVDs are prominent and are a leading cause of death in smokers (Al Suwaidi et al., 2012; Bhatnagar et al., 2019; Pratiti & Mukherjee, 2019; Rezk-Hanna & Benowitz, 2019; Selim et al., 2013; Sibai et al., 2014). Since narghile tobacco smoke contains constituents, “similar” to those generated by cigarettes (Ben Saad, 2009), narghile use could lead to similar central and/or peripheral cardiovascular abnormalities (Alomari et al., 2014; Ben Saad, 2009; Bhatnagar et al., 2019; Rezk-Hanna & Benowitz, 2019). Several studies have been interested in the effects of narghile use on the cardiovascular system at rest (Al-Kubati et al., 2006; Alomari et al., 2014, 2015; Al Suwaidi et al., 2012; Bentur et al., 2014; Bhatnagar et al., 2019; Cobb et al., 2012; El-Zaatari et al., 2015; Hawari et al., 2013; Nelson et al., 2016; Pratiti & Mukherjee, 2019; Rezk-Hanna & Benowitz, 2019; Selim et al., 2013; Shaikh et al., 2008; Sibai et al., 2014). These studies reported an association of short- and long-term narghile use with increased CVDs risk and severity as well as mortality (Al-Kubati et al., 2006; Alomari et al., 2014, 2015; Al Suwaidi et al., 2012; Bhatnagar et al., 2019; Cobb et al., 2012; Hawari et al., 2013; Nelson et al., 2016; Rezk-Hanna & Benowitz, 2019; Selim et al., 2013; Shaikh et al., 2008; Sibai et al., 2014). Short-term narghile use affects the heart rate (HR), blood pressure, baroreflex sensitivity, tissue oxygenation, and vascular function (Al-Kubati et al., 2006; Alomari et al., 2014, 2015; Bhatnagar et al., 2019; Cobb et al., 2012; Hawari et al., 2013; Nelson et al., 2016; Shaikh et al., 2008). First, narghile use induces an immediate and transient increase in HR, and systolic and diastolic blood pressure (SBP and DBP, respectively) (Al-Kubati et al., 2006; Alomari et al., 2014; Bentur et al., 2014; El-Zaatari et al., 2015; Pratiti & Mukherjee, 2019; Shaikh et al., 2008), and a decrease in HR variability (Cobb et al., 2012). Second, narghile use affects vascular function (Alomari et al., 2014, 2015), vascular resistance (Alomari et al., 2014), forearm blood flow (Alomari et al., 2014), and venous outflow (Alomari et al., 2014). Third, narghile use induces an increase in myocardial oxygen demand (Nelson et al., 2016). Long-term narghile use has been associated with cardiovascular abnormality (Al Suwaidi et al., 2012; Selim et al., 2013; Shafique et al., 2012; Sibai et al., 2014). Compared to non-smokers, narghile smokers had higher HR and blood pressure (Selim et al., 2013; Shafique et al., 2012), including increased risks of hypertension (Shafique et al., 2012) or coronary artery diseases (Al Suwaidi et al., 2012; Selim et al., 2013; Sibai et al., 2014). Since exploring the chronic effects of narghile use at rest may fail to demonstrate its real impact on cardiovascular function, some authors have tested its chronic effects on exercise capacity measures. Exploring the physiological response during exercise may detect subtler effects that can emerge only during stressful/dynamic conditions of narghile use on the cardiovascular system (ATS/ACCP, 2003).
To the finest of the authors’ knowledge, no previous review has raised the issue of the chronic effects of narghile use on cardiovascular response during exercise. Therefore, this systematic review aimed at assessing the most up-to-date published literature in order to evaluate the chronic effects of narghile use on cardiovascular response during exercise.
Methods
Protocol and Eligibility Criteria
This systematic review followed the preferred reporting items for systematic reviews guidelines (Moher et al., 2009). Original articles written in English and evaluating the chronic effects of narghile use on cardiovascular response during exercise were considered. No restrictions were applied in terms of study design, setting, country, or time frame. Publications not in compliance with this systematic review purpose as well as those not representing original research (i.e., reviews, editorials, qualitative papers, case reports, case-series, and letters to editors) were excluded.
Information Sources and Search
PubMed and Scopus were searched irrespective of time limits or filters. The final search was completed on January 31, 2020. For PubMed, the search was carried out using a strategy employing the combination of synonyms of “narghile” and terms related to “exercise” (Box 1). For Scopus, the previous terms were searched in the article title or abstract or keywords. Only studies performed on humans were included. In addition, the reference lists of the included articles were checked. Both authors of this review agreed on the articles to be included in this systematic review. All the aspects of systematic review methods were specified before commencing the review. The aspects comprised the following points: studies’ inclusion criteria, search strategy, screening method, abstraction, and data analysis. The design aspect was planned to minimize the effects of a possible bias.
Box 1.
Terms applied in the present systematic review.
| Synonyms of “narghile” | Terms related to “exercise” | ||
|---|---|---|---|
| Terms related to “walk test” | Terms related to “cardiopulmonary exercise test” | Others | |
| Arghileh[Tiab] OR Argil[Tiab] OR Argileh[Tiab] OR
Borry[Tiab] OR Chicha[Tiab] OR Chichi[Tiab] OR Chilam[Tiab]
OR Ghelyan[Tiab] OR Goza[Tiab] OR Guza[Tiab] OR Hooka[Tiab]
OR Hookah[Tiab] OR Hubble Bubble[Tiab] OR Hubble-Bubble[Tiab] OR Hukka[Tiab] OR Huqqa[Tiab] OR Narghile[Tiab] OR Narguile[Tiab] OR Narguilé[Tiab] OR Narguileh[Tiab] OR Sheesha[Tiab] OR Shisha[Tiab] OR Water Pipe[Tiab] OR Water-Pipe[Tiab] OR Waterpipe[Tiab] |
“Submaximal Exercise capacity”[Tiab] OR “Submaximal
Test”[Tiab] OR “Test 6 Minute Walk”[Tiab] OR “six minute walktest”[tiab] OR “six minutewalking distance”[tiab] OR “six-min walk distance”[tiab] OR “six-min walktest”[tiab] OR “six minute walk distance”[tiab] OR “six-minute walk distance”[tiab] OR “six-minute walkingdistance”[tiab] OR “six-minute walktest”[tiab] OR “six-minute walk”[tiab] OR “Walk Test”[MeSH] OR “walk test”[tiab] OR “walking”[MeSH] OR”6-min walk distance”[tiab] OR “6-min walk test”[tiab] OR “6-minute walk test”[tiab] OR “6-minute walk distance”[tiab] OR “6-min walk”[tiab] OR “6MWD”[tiab] OR “6MWT”[tiab] |
“Bicycle Ergometry Test”[Tiab] OR “Cardiopulmonary Exercise Test”[Tiab] OR “Cardiorespiratory Fitness”[Tiab] OR “Cardiopulmonary Exercise Testing”[Tiab] OR “Exercise Testing”[Tiab] OR “Exercise Tests”[MeSH] OR “Fitness Testing”[Tiab] OR “Maximal Exercise capacity”[Tiab] OR “Treadmill Test”[Tiab] OR “Physical Fitness Testing”[Tiab] |
“Cardiomegaly, Exercise-Induced”[Mesh] OR “Circuit-Based
Exercise”[Mesh] OR “Cool-Down Exercise”[Mesh] OR “Exercise Movement Techniques”[Mesh] OR “Exercise capacity”[Tiab] OR “Exercise Therapy”[Mesh] OR “Exercise Tolerance”[Mesh] OR “Exercise”[Mesh] OR “High-Intensity Interval Training”[Mesh] OR “Muscle Stretching Exercises”[Mesh] OR “Physical activity”[Tiab] OR “Plyometric Exercise”[Mesh] OR “Post-Exercise Hypotension”[Mesh] OR “Resistance Training”[Mesh] OR “Warm-Up Exercise”[Mesh] OR “Training”[Tiab] |
Study Selection
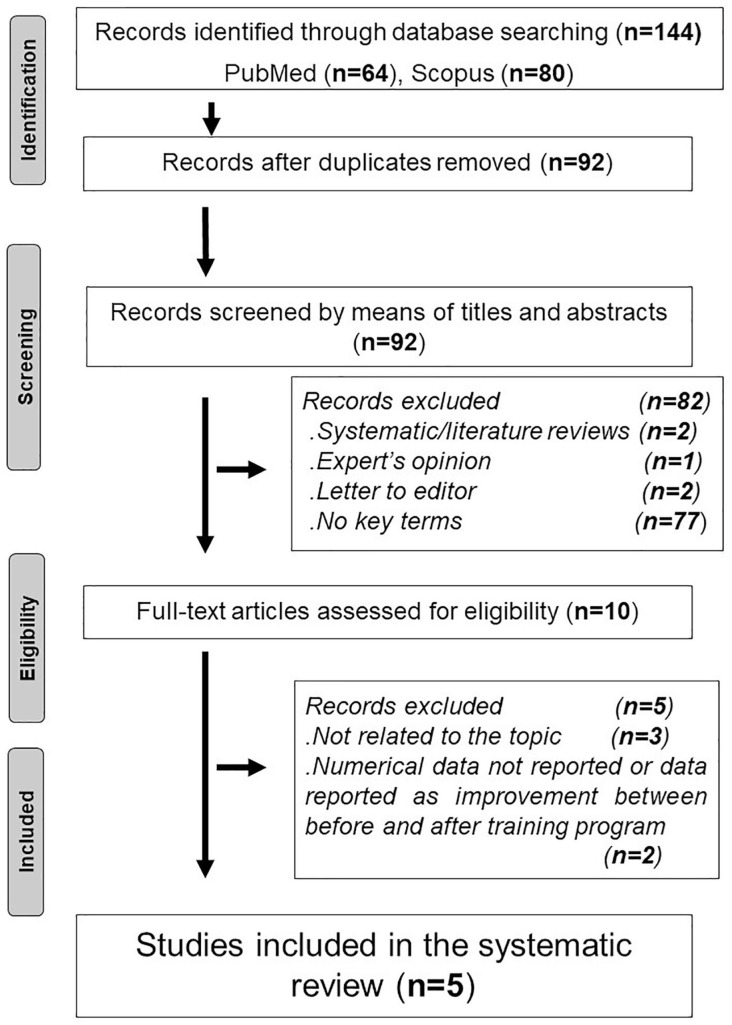
The process of article selection is outlined in Figure 1. Duplicate articles were eliminated using End-Note X9 library. Articles were eliminated if they had duplicate titles, did not contain the keywords, or included the words “review,” “expert’s opinion,” or “qualitative.” Abstracts of the remaining articles were scanned for the search terms and the words “review,” “expert’s opinion,” and “qualitative.” Full texts of the remaining articles were read to check for their relevance to the searched topics. Next, the full texts of the selected studies were screened, and disagreements concerning eligibility were again resolved by consensus.
Figure 1.
Process of article selection.
Data Collection Process
The two authors collected data independently, using a pilot-tested extraction form and resolved disagreements by consensus. The extracted data included the main methodological characteristics of the articles: study data (years of realization and publication, place, design, recruitment mode, sample size calculation, inclusion, non-inclusion and exclusion criteria, main collected data and outcomes, applied protocols, statistical methods and comparison with control groups, narghile use data [name, exclusive character, starting age, years of smoking, types of narghile-tobacco, way of quantification, levels of used tobacco, gram of tobacco/narghile session, last narghile use]) and participant data (number, age, sex). The extracted data also included the articles’ main results. Strengths as well as flaws associated with the studies’ methodology were discussed. The results were presented in the context of all other available evidence.
Results
Study Selection
The search process yielded 64 articles (Figure 1). Seven articles related to the chronic effects of narghile use on cardiovascular response during exercise were found (Ben Saad et al., 2014; Hawari et al., 2017; Koubaa et al., 2019; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a, 2015b). Screening of the seven articles’ reference lists did not find any additional papers. Among the aforementioned seven papers, two were not retained since data were expressed as improvement between before and after training program (Koubaa et al., 2019; Koubaa, Triki, Trabelsi, et al., 2015b). While the authors of the first article reported no significant difference in global and cardiovascular responses between narghile smokers, cigarette smokers, and healthy non-smokers, without noting any numerical data (Koubaa, Triki, Trabelsi, et al., 2015b), those of the second article (Koubaa et al., 2019) did not compare the cardiovascular data of the three groups (narghile smokers, cigarette smokers, and healthy non-smokers). Table 1 exposes the main methodological characteristics of the five retained studies. Table 2 exposes the main results of the four studies opting for the use of a cardiopulmonary exercise test (CPET) (Hawari et al., 2017; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a).
Table 1.
Methodological Characteristics of the Published Studies Aiming at Evaluating the Chronic Effects of Narghile Use on Cardiovascular Response During Exercise.
| Authors | Ben Saad et al. (2014) | Koubaa, Triki, Trabelsi, Baati, et al. (2015) | Koubaa, Triki, Trabelsi, et al. (2015a) | Koubaa, Triki, Trabelsi, Masmoudi, et al. (2015) | Hawari et al. (2017) |
|---|---|---|---|---|---|
| Yr of publication | 2014 | 2015 | 2015 | 2015 | 2017 |
| Yr of the study | 2010 | NR | NR | NR | 2013–2015 |
| Town (Country) | Sousse (Tunisia) | Sfax (Tunisia) | Sfax (Tunisia) | Sfax (Tunisia) | Amman (Jordan) |
| Name of the smoking mode | Narghile | Hookah | Hookah | Hookah | Waterpipe |
| Recruitment method |
ENSs: (a) database of ENSs who had participated
in previous studies; (b) announcement in a
newspaper CG: local staff of faculty or hospital and acquaintances |
Public function (profession does not required physical exertion) | Public function (profession does not required physical exertion) | NR | Announcement in universities |
| Inclusion criteria | Males >5 NY 20–60 Yrs ENSs |
Males >5 HY Healthy Sedentary |
Males >5 HY Healthy Sedentary |
Males >5 HY Healthy Sedentary |
Males >3 times/week for the past 3 Yrs Healthy 18–26a Yrs |
| Non-inclusion criteria | 6MWT contraindications (ATS,
2002) Current or former cigarette or pipe smoking Asthma Recent respiratory infection Diabetes mellitus >5 Yrs Rheumatologic, orthopedic, or surgical diseases interfering with walking Chronic medication-use (beta-blockers, diuretics, corticosteroids) |
Non-approval of cardiologist
physician Obesity Use of nutritional supplements or medications |
Non-approval of cardiologist
physician Obesity Use of nutritional supplements or medications |
Dyslipidemia Obesity |
Strenuous exercises or activities48 h prior to the
visit Other form of tobacco use |
| Non-inclusion criteria | Imperfect realization of required maneuvers of
spirometry Inability to perform the 6MWT exactly |
||||
| Exclusion criteria | NR | Dyslipidemia RA or EP during the previous 12 months |
Dyslipidemia RA or EP during the previous 12 months FEV1/FVC < 70% |
Use of nutritional supplements or medications RA or EP during the previous 12 months |
Chronic medication use Illicit drug use BMI ≥40 kg/m2 Chronic medical conditions Abnormal heart rhythm HRRest > 100 or < 50 bpm Blood pressures (>140/90 mmHg) Oxy-satRest < 95% |
| Exclusive narghile smoking | Yes | NR | NR | NR | Yes |
| Number of ENSs |
Cross-sectional: 70 Comparative study: 25 |
12 | 14 | 14 | 69 |
| Age (Yrs) |
Cross-sectional: 20–60a, 32 (26–43)b
Comparative study: 40–60a, 47 ± 6c |
44.0 ± 1.7c | 43.7 ± 2.3c | 43.7 ± 2.3c | 22.1 (19.0–26.1)e |
| Way of quantification | NY kg of cumulated tobacco |
HD HY kg of cumulated tobacco |
HY kg of cumulated tobacco |
HY kg of cumulated tobacco |
HW |
| Levels of used tobacco |
Cross-sectional: 17 (8–32)b NY 5–145a NY 151 (73–292)b kg 46–1323a kg Comparative study: 46 ± 37c NY* |
>5 HY | >5 HY | >5 HY | 8.9d HW |
| Last narghile (h) | At least 24 | NR | NR | NR | At least 48 |
| Gram of tobacco/narghile session | 20–30a
25d |
10–25a | 10–25a | 10–25a | NR |
| Performed exercise tests (guidelines) | 6MWT (ATS, 2002) | CPET, treadmill (NR) | CPET, treadmill (NR) | CPET, treadmill (NR) | CPET, ergocycle (ATS/ACCP, 2003) |
| Other tests | Spirometry Electrocardiogram Physical activity questionnaire (Voorrips et al., 1991) |
Antioxydant data Lipid blood data |
Spirometry | Antioxidant data Lipid blood data |
Plethysmography |
| Main collected data related to the aims of this systematic review | HR (bpm; % PMHR) SBP (mmHg) DBP (mmHg) 6MWD (m, % predicted (Ben Saad et al., 2009; Chetta et al., 2006)) Oxy-sat (%) Dyspnea (mMRC (Mahler & Mackowiak, 1995), VAS (Sergysels & Hayot, 1997)) Number of stops Estimated cardiorespiratory and muscular chain’ age (Ben Saad et al., 2009) |
HR (bpm) SBP (mmHg) DBP(mmHg) O2max (mL/min/kg) |
HR (bpm) SBP (mmHg) DBP (mmHg) vO2max (km/h) O2max (mL/min/kg) RI |
O2max (mL/min/kg) | HR (bpm, % PMHR) SBP (mmHg) DBP (mmHg) O2max (ml/min/kg, %) HR/O2 (beats/ml/kg) O2 pulse (%) HRR (bpm) Work (Watt, %) (ATS/ACCP, 2003) O2/Work (ml/min/watt) BR (%) Exercise time (min) E (%) E/O2 (%) E/CO2 (%) Leg fatigue (Borg, 1982) Dyspnea (Borg, 1982) AT (% - peak O2max) |
| Main outcomes | Lung function 6MWD |
Antioxidant defense capability Lipid profile |
Lung function Cardiorespiratory fitness |
Antioxidant defense Lipid profile |
Lung function Exercise capacity |
| Comparison with CSs | NA |
n = 11 CSs 45.5 ± 1.7c ≥ 10 PY |
n = 15 CSs 43.2 ± 2.1c ≥10 PY |
n = 15 CSs 43.2 ± 2.1c ≥10 PY |
NA |
| Comparison with HNSs |
n = 53 40–60a Yrs 49 ± 5c Yrs |
n = 12 44.5 ± 1.3c |
n = 14 43.8 ± 2.1c |
n = 12 43.8 ± 2.1c |
n = 69 21.4 (18.2–26.4)e* |
Note. AT = anaerobic threshold; BMI = body mass index; bpm = beats per minute; BR = breathing reserve; CG = control group; CPET = cardiopulmonary exercise test; CS = cigarette smoker; DBP = diastolic blood pressure; ENS = exclusive narghile smoker; EP = exercise program; FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity; h = hour; HD = hookah-day; HNS = healthy non-smoker; HR = heart rate; HRR = heart rate reserve; HW = heads-week; HY = hookah-years; min = minute; mMRC = modified Medical Research Council scale; n = number; NA = non applied; NR = not reported; NY = narghile-years; O2 = oxygen; Oxy-sat = oxyhemoglobin saturation; PMHR = predicted maximal heart rate; PY = pack-years; RA = regular physical activity; Rest = at rest; RI = recovery index; SBP = systolic blood pressure; VAS = visual analog scale; Yr = year. CO2 = carbon dioxide production; E = ventilation minute; O2 = oxygen uptake; O2max = maximal oxygen uptake; vO2max = velocity at maximum oxygen uptake; 6MWD = 6-min walk distance; 6MWT = 6-min walk test.
Data were: aRange (minimum–maximum), bMedian (IQR), cMean ± SD. dMean, eMean (minimum–maximum).
Study of Ben Saadet al.: *p < .05: ENSs vs. HNSs.
Studies of Koubaa et al.: *p < .05: ENSs vs. HNSs.
Study of Hawari et al.: *p < .05: ENSs vs. HNSs.
Table 2.
Main Results of the Published Studies Aiming at Evaluating the Chronic Effects of Narghile Use on Cardiovascular Response During Exercise.
| Authors | Global Response | Cardiovascular Response | Conclusion | ||
|---|---|---|---|---|---|
| ME | PE | Rest | PE | ||
| Koubaa, Triki, Trabelsi, Baati, et al., 2015 | NR | O2max (mL/min/kg): 36.6 ± 1.2# | HR (bpm): 93 ± 4*
SBP (mmHg): 138 ± 3* DBP (mmHg): 86 ± 4 |
NR | The ENS group had a lower O2max than the CS group (38.9 ± 2.5 mL/min/kg) and higher resting HR and SBP than the HNS group (78 ± 4 bpm, 131 ± 3 mmHg, respectively). |
| Koubaa, Triki, Trabelsi, et al., 2015a | .NR |
O2max (mL/min/kg): 34.3 ±
0.8#*
VO2max (km/h): 9.8 ± 0.2*# |
HR (bpm): 91 ± 3*
SBP (mmHg): 143 ± 5*¥ DBP (mmHg): 93 ± 3* |
RI: 16 ± 1*# | The ENS group had lower O2max, VO2max, and RI than the CS (35.8 ± 0.9 mL/min/kg, 10.3 ± 0.2 km/h, and 17 ± 1, respectively) and HNS groups (39.0 ± 0.7 mL/min/kg, 11.2 ± 0.2 km/h, and 18 ± 1, respectively) and had higher resting HR, SBP, and DBP than the HNS group (82 ± 5 bpm, 124 ± 7 mmHg, and 84 ± 7 mmHg, respectively). |
| Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015 | NR | O2max (mL/min/kg): 34.3 ± 0.8#* | NR | NR | The ENS group had lower O2max than the CS and HNS groups (35.8 ± 0.9, 39.0 ± 0.7 mL/min/kg, respectively). |
| Hawari et al., 2017 | Dyspnea: 2.9 ± 1.4*
Leg fatigue: 11.7 ± 2.2* |
O2max (%): 57.5 ±
11.2*
Work (%): 60.0 ± 8.7* O2/Work (mL/min/W): 12.9 ± 3.2 E/O2(%): 22.3 ± 4.8 E/CO2(%): 25.1 ± 2.6* Dyspnea: 5.4 ± 1.6* Leg fatigue: 15.3 ± 2.6 Exercise time (min): 8.5 ± 1.0* |
NR | HR (%): 87 ± 9.0%*
HRR (bpm): 25.3 ± 15.8* HR/O2 (beats/mL/kg): 2.8 ± 0.7 HR/O2 (%): 81.3 ± 16.0 SBP (mmHg): 144.1 ± 17.1 DBP (mmHg): 79.4 ± 14.8 |
Compared to the HNS group, the ENS group had lowerO2max (61.2 ± 10.1%), work (63.6 ± 12.0%), exercise time (8.9 ± 1.2 min), HR (93 ± 6%), but had higher HRR (14.6 ± 10.6 bpm), dyspnea at ME and PE (1.8 ± 0.98, 3.9 ± 1.4, respectively), leg fatigue at ME (10.7 ± 1.9), and E/CO2 (23.8 ± 2.7%). |
Note. bpm = beats per minute; DBP = diastolic blood pressure; CS = cigarette smoker; ENS = exclusive narghile smoker; HNS = healthy non-smoker; HR = heart rate; HRR = heart rate reserve; ME = mid-exercise; min = minute; NR = not reported; PE = peak exercise; RI = recovery index; SBP = systolic blood pressure; CO2 = dioxide carbon production; E = minute ventilation; O2 = oxygen uptake;O2max = maximum oxygen uptake; vO2max = velocity at maximum oxygen uptake.
All data were mean ± SD.
Studies of Koubaa et al.: *p < .05: ENSs vs. HNSs; #p < .05: ENSs vs. CSs.
Study of Hawari et al.: *p < .05: ENSs vs. HNSs.
Study Characteristics
The studies were published between 2014 and 2017 (Table 1). Four studies included Tunisian adults (Ben Saad et al., 2014; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a) and one included Jordanian young adults (Hawari et al., 2017). All the studies opted for a cross-sectional comparative design. One study (Ben Saad et al., 2014) included two parts: cross-sectional and comparative studies. Two studies (Ben Saad et al., 2014; Hawari et al., 2017) included one control group of healthy non-smokers. The remaining three studies (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a) included two control groups of healthy non-smokers and cigarette smokers (exclusive cigarette smokers or not). No study opted to blind the investigators and/or the technicians to the groups. All the retained studies included convenience samples. The narghile smokers’ recruitment method was not reported in one study (Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015). In the remaining four studies, the following methods were applied to recruit narghile smokers: participants’ database in previous studies (Ben Saad et al., 2014), announcement in newspapers (Ben Saad et al., 2014) or universities (Hawari et al., 2017), and persons from public function (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a). The control groups’ recruitment methods were reported only in one study (i.e., local staff in a faculty or hospital and acquaintances) (Ben Saad et al., 2014).
Only males were included in the retained studies. The following additional inclusion criteria were applied to narghile smokers: good health (Hawari et al., 2017; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), age range 20–60 (Ben Saad et al., 2014) or 18–26 (Hawari et al., 2017) years, narghile use > 5 narghile-years (Ben Saad et al., 2014; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a) or >3 times/week for the past 3 years (Hawari et al., 2017), and exclusive narghile-smoker traits (Ben Saad et al., 2014; Hawari et al., 2017; Rostagno & Gensini, 2008). The following non-inclusion and exclusion criteria were applied: contraindications of the 6-min walk test (6MWT) (Ben Saad et al., 2014), some chronic (e.g., asthma [Ben Saad et al., 2014], mellitus diabetes [Ben Saad et al., 2014], obesity [Hawari et al., 2017; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a], dyslipidemia [Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a], abnormal heart rhythm or HR [Hawari et al., 2017], arterial hypertension [Hawari et al., 2017]) or acute (e.g., recent respiratory infection [Ben Saad et al., 2014]) conditions or symptoms (Ben Saad et al., 2014; Hawari et al., 2017; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), diseases interfering with exercise (Ben Saad et al., 2014), chronic medications use (Ben Saad et al., 2014; Hawari et al., 2017; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), nutritional supplements use (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), lack of cooperation during tests (Ben Saad et al., 2014), illicit drug use (Hawari et al., 2017), non-approval of a cardiologist (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), acute (e.g., strenuous exercise or activities 24 hr [Ben Saad et al., 2014] or 48 hr [Hawari et al., 2017] prior to the visits) or chronic (e.g., regular physical activity or exercise program during the previous 12 months [Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a]) exercise/activities, ventilatory obstructive defect (Koubaa, Triki, Trabelsi, et al., 2015a), and resting oxyhemoglobin saturation (Oxy-sat) <95% (Hawari et al., 2017).
Smoking Data
The name of the tobacco used for narghile was different in the five studies: narghile (Ben Saad et al., 2014), hookah (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), and waterpipe (Hawari et al., 2017) (Table 1). No study reported the starting age of narghile use. Only one study reported the mean (minimum–maximum) of smoking years (i.e., 4.9 [3–8] years [Hawari et al., 2017]). Only one study reported the type of narghile tobacco (i.e., Tabamel [Moassel] [Ben Saad et al., 2014]). The quantification ways of narghile use were different in the five studies: narghile-years (Ben Saad et al., 2014), hookah-day (Koubaa, Triki, Trabelsi, Baati, et al., 2015), hookah-years (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), heads-week (Hawari et al., 2017), and kg of cumulated tobacco (Ben Saad et al., 2014; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a). The quantity (in gram) of tobacco per narghile session was different in the studies: 20–30 g (Ben Saad et al., 2014) or 10–25 g (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a). The levels of narghile tobacco used were large and different in the five studies: >5 hookah-years (Ben Saad et al., 2014; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), 5–145 narghile-years (Ben Saad et al., 2014), median of 17 narghile-years (Ben Saad et al., 2014), means of 46 narghile-years (Ben Saad et al., 2014) or 8.9 heads-week (Hawari et al., 2017). The time of last narghile use was reported only in two studies (Ben Saad et al., 2014; Hawari et al., 2017). In the three studies involving cigarette smokers, the amount of consumed tobacco was ≥10 pack-years (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a).
Participants’ Characteristics
Only one study calculated the needed sample size (Ben Saad et al., 2014) (Table 1). The sample sizes of narghile smokers, healthy non-smokers, and cigarette smokers varied from 12 to 70, 12 to 69, and 11 to 15, respectively. The total number of participants was 380 (179 narghile smokers, 160 healthy non-smokers, and 41 cigarette smokers). The ages of narghile smokers, healthy non-smokers, and cigarette smokers varied from 19 to 60, 18 to 60, and 43 to 45 years, respectively.
Applied Tests
The applied tests were different in the five studies (Table 1). One study applied a low tech test (i.e., a field test: 6MWT) (Ben Saad et al., 2014). The four remaining studies opted for a high tech test (i.e., CPET) (Hawari et al., 2017; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a) using a treadmill (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a) or a bicycle ergometer (Hawari et al., 2017). The following data/tests were reported/applied: anthropometric data (Ben Saad et al., 2014; Hawari et al., 2017; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), clinical data (Ben Saad et al., 2014), physical activity questionnaire (Ben Saad et al., 2014), electrocardiogram (Ben Saad et al., 2014), spirometry (Ben Saad et al., 2014; Koubaa, Triki, Trabelsi, et al., 2015a), plethysmography (Hawari et al., 2017), antioxidant markers (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015), and lipid blood data (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015).
Collected Data
During the 6MWT, the main outcome was the 6-min walk distance (6MWD) (Ben Saad et al., 2014). The following data were collected at rest (Rest) and at the end (End) of the walk: HR, blood pressures, Oxy-sat (%), and dyspnea (visual analog scale). During CPET, the collected data were divided into four categories (general response/exercise tolerance, cardiovascular, ventilatory, and muscular responses) based on the most applied strategy to interpreted CPET (Aguilaniu & Wallaert, 2013). For the general response/exercise tolerance, the main outcome was the maximal oxygen uptake (O2max). The latter was expressed in mL/min/kg (Hawari et al., 2017; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a) and/or as a percentage of the predicted O2max (Hawari et al., 2017). Concerning the cardiovascular response, the main outcomes were HR expressed in bpm (Ben Saad et al., 2014; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a) or as a percentage of the predicted maximal HR (PMHR; Ben Saad et al., 2014; Hawari et al., 2017), and blood pressure (mmHg) (Ben Saad et al., 2014; Hawari et al., 2017; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a). For the ventilatory response, three outcomes were reported: Oxy-sat (%) (Ben Saad et al., 2014), ventilation minute (E, %) (Hawari et al., 2017), and breathing reserve (%) (Hawari et al., 2017). For the muscular response, the main outcome was the anaerobic threshold (%) (Hawari et al., 2017). Additional collected data related to the 6MWT and CPET are detailed in Table 1 and in the online Appendix.
Chronic Effects of Narghile Use on 6MWT Data
Only one study evaluated the chronic effects of narghile use on 6MWT (Ben Saad et al., 2014). For exclusive narghile smokers, the study’s main findings were:
i) The 6MWD mean was 101 ± 14%, and 20% of exclusive narghile smokers had an abnormal 6MWD (i.e., < lower limit of the normal);
ii) The factors that significantly influenced 6MWD, explaining 38% of its variability, were body mass index (BMI, kg/m2), forced expiratory volume in one second (FEV1, L), and narghile use (in narghile-years);
iii) 20% of exclusive narghile smokers had dyspneaRest (modified Medical Research Council scale), 9% had a high dyspneaEnd score (>5/10, visual analog scale), but no one discontinued the walk test or required a rest during the walk;
iv) The means ± SD of the HRRest and HREnd in the group of exclusive narghile smokers were, respectively, 40 ± 5% and 66 ± 12%, and 34% of exclusive narghile smokers had a low HREnd (<60%);
v) The medians (interquartile) of SBPRest, SBPEnd, DBPRest, and DBPEnd were 120 (120–130), 150 (140–160), 80 (70–85), and 90 (80–100) mmHg, respectively;
vi) The medians (interquartile) of Oxy-satRest and Oxy-satEnd were 98% (97–98) and 98% (97–98), respectively, and 3% of exclusive narghile smokers had an Oxy-sat decrease of >5 points during the test;
vii) Exclusive narghile smokers had significantly higher estimated cardiorespiratory and muscular chain age than the chronological age (65 ± 12 vs. 47 ± 6 years, respectively) (Ben Saad et al., 2014);
Compared to healthy non-smokers, a subgroup of exclusive narghile smokers had significantly lower scores of sporting, leisure, and physical activities; lower HREnd, SBPEnd, Oxy-satRest, and 6MWD (98 ± 7 vs. 87 ± 9%, respectively); higher BMI, dyspneaRest, dyspneaEnd, HRRest, DBPRest, and DBPEnd; higher percentages of participants with sedentary status; and higher estimated cardiorespiratory and muscular chain age (69 ± 11 vs. 51 ± 11 years, respectively), but they had similar spirometric data. The authors concluded that “narghile use may play a role in reducing submaximal aerobic capacity.”
Chronic Effects of Narghile Use on CPET Data
At peak exercise, exclusive narghile smokers had lower O2max compared to cigarette smokers (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a) or healthy non-smokers (Hawari et al., 2017; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a; Table 2). At rest, compared to healthy non-smokers, exclusive narghile smokers had higher HR (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), SBP (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), and DBP (Koubaa, Triki, Trabelsi, et al., 2015a). At peak exercise, compared to healthy non-smokers, exclusive narghile smokers had lower HR (Hawari et al., 2017). No study reported resting ventilatory data. At peak exercise, one study reported no significant difference between exclusive narghile smokers and healthy non-smokers in breathing reserve (51.7 ± 9.7 vs. 49.4 ± 12.5%, respectively), E (53.7 ± 10.6 vs. 56.1 ± 13.2%, respectively), and anaerobic threshold (33.6 ± 10.4 vs. 32.8 ± 8.6% of peakO2) (Hawari et al., 2017). Additional results are highlighted in Table 2 and in the online Appendix.
Discussion
The take-home message derived from this systematic review including five studies was that narghile use is associated with reduced submaximal and maximal aerobic capacities, and with abnormal cardiovascular status at rest, at the end of the exercise and during the recovery period. It appears that this is the first systematic review that treats the topic of the chronic effects of narghile use on cardiovascular response during exercise. Narghile use has been linked with numerous cardiovascular effects that influence or contribute to the deterioration of the overall health status (Azar et al., 2016; Qasim et al., 2019). Regrettably, notwithstanding studies recording cardiovascular disease risks related with narghile use, people remain to assume that this smoking mode is safer than cigarettes, primarily due to being unconscious of its harmful health effects (Qasim et al., 2019). Narghile alertness should be systematic and more vigorous (Ben Saad, 2009; Qasim et al., 2019).
Discussion of Results
Chronic Effects of Narghile Use on 6MWT Data (6MWD, HR, Blood Pressure, Dyspnea)
Narghile use had a negative impact on submaximal aerobic capacity (i.e., abnormal 6MWD, signs of walking intolerance [desaturation, dyspneaEnd], chronotropic insufficiency) in the group of exclusive narghile smokers (Ben Saad et al., 2014). In the univariate model, narghile use explained 13% of 6MWD variability (Ben Saad et al., 2014). Another important strategic result was that narghile use accelerated cardiorespiratory and muscle chain aging (Ben Saad et al., 2014). This is an unwavering argument to motivate narghile smokers to stop smoking.
How did Ben Saad et al. (2014) explain the 6MWD impairment? The following three factors, derived from a multivariate analysis, were advanced to explain the 6MWD impairment: BMI, resting spirometric data, and quantity of narghile use. First, BMI appears to be the main predictor of 6MWD decline (alone it explains 28% of 6MWD variability: 6MWD decreased by approximately 5 m when BMI increased by one unit [Ben Saad et al., 2014]). The above result confirms once again that obesity is a predictive factor for 6MWD decline (ATS, 2002; Kammoun & Ben Saad, 2020). Second, resting spirometric data (e.g., FEV1) had a negative impact on 6MWD and they doubled 6MWD decline ( ~ 25 m) in exclusive narghile smokers (Ben Saad et al., 2014). In fact, spirometric data are predictors of 6MWD (ATS, 2002). Exclusive narghile smokers with an abnormal 6MWD had significantly lower FEV1 compared to those with a normal 6MWD. Alteration of the resting spirometric data may partly explain the decline of 6MWD in exclusive narghile smokers (Ben Saad et al., 2014). Third, narghile use appears as a factor in 6MWD decline (Ben Saad et al., 2014). On the one hand, a higher quantity of tobacco used resulted in a lower 6MWD. On the other hand, exclusive narghile smokers with an abnormal 6MWD were significantly more engaged in narghile use compared to those with a normal 6MWD (55 ± 44 vs. 19 ± 16 narghile-years, respectively). This decline in 6MWD can be explained by the negative consequences of narghile use on the cardiorespiratory and muscular functions (Ben Saad et al., 2014).
At rest, the subgroup of exclusive narghile smokers had significantly higher HRRest and DBPRest than the subgroup of healthy non-smokers (Ben Saad et al., 2014). These results are in line with the findings of previously published studies, evaluating the effect of narghile use on resting cardiovascular data (Al-Kubati et al., 2006; Alomari et al., 2014; Bentur et al., 2014; El-Zaatari et al., 2015; Pratiti & Mukherjee, 2019; Shaikh et al., 2008). Short-term and long-term narghile use has been reported to be associated with an increase in HR, SBP, and DBP (Al-Kubati et al., 2006; Alomari et al., 2014; Bentur et al., 2014; El-Zaatari et al., 2015; Pratiti & Mukherjee, 2019; Shaikh et al., 2008). Narghile smoke contains high levels of nicotine (Ben Saad, 2009). The systemic hemodynamic effects of nicotine are mediated primarily by activation of the sympathetic nervous system (Benowitz & Burbank, 2016). Nicotine releases norepinephrine from adrenergic neurons and increases adrenal release of epinephrine (Benowitz & Burbank, 2016). Narghile use acutely increases HR and blood pressures, an effect mediated by nicotine-induced beta-adrenergic stimulation (Bhatnagar et al., 2019). Long-term narghile use has been associated with higher HR and blood pressures (when comparing exclusive narghile smokers with non-smokers) (Selim et al., 2013; Shafique et al., 2012). The chronic effect of narghile use can be explained by two interchangeable mechanisms: (a) disturbance in cardiac autonomic control with long-term reduction in vagal drive (Hayano et al., 1990; Lucini et al., 1996); and/or (b) chronic increase in resting muscle sympathetic nerve activity as shown in cigarette smokers (Hering et al., 2010). In narghile smokers, the “abnormal” resting cardiovascular status can partially explain the submaximal aerobic capacity decline (Kokkinos et al., 2009). At peak exercise, 34% of exclusive narghile smokers had chronotropic insufficiency with lower HR and SBP in the subgroup of exclusive narghile smokers compared to the subgroup of healthy non-smokers (Ben Saad et al., 2014). This may reflect the effect of narghile use on the cardiac autonomic function with abnormal sympathetic control (Cobb et al., 2012). All these data highlight the negative impact of narghile use on cardiovascular adaptation to exercise.
Exclusive narghile smokers had a lower tolerance to exercise (higher dyspneaEnd scores, important desaturation) than heathy non-smokers (Ben Saad et al., 2014). This limitation in exercise capacity can partially be explained by the negative impact of narghile use on resting breathing frequency and spirometric data (Ben Saad et al., 2014).
Chronic Effects of Narghile Use on CPET Data
Global response
Narghile use is associated with reduced maximal exercise capacity in the retained studies (Table 2). First, narghile smokers generated lower O2maxvalues than healthy non-smokers (Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a) or cigarette smokers (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a). Second, narghile smokers had a significantly shorter exercise time compared to healthy non-smokers (Hawari et al., 2017). Third, compared to healthy non-smokers, narghile smokers reported higher perception of leg fatigue and dyspnea at mid exercise, and dyspnea at peak exercise (Hawari et al., 2017).
How can the negative effect of narghile use on O2max be explained? The first hypothesis could be the “possible” cumulated negative effects of acute impacts of narghile use on maximal exercise capacity/endurance (Hawari et al., 2013). According to Hawari et al. (2013), the acute harmful effects could be related to the reduced capacity for carrying oxygen due to the increase in carbon monoxide levels. The second hypothesis can be extrapolated from the effect of cigarette smoking on exercise capacity (Muller et al., 2019). Muller et al. (2019) identified abnormalities in the cardio-circulatory and respiratory chain, supporting oxygen delivery to muscle.
Narghile use negatively influenced the breathing efficiency as, compared to healthy non-smokers, narghile smokers produced significantly higher ratio betweenE and carbon dioxide production (Hawari et al., 2017). The following main factors can be incriminated in breathing efficiency: airway obstruction, hyperinflation, increased dead space ventilation, abnormal resting hemodynamic, and muscle endurance (Hawari et al., 2017). According to Hawari et al. (2017), muscle fatigue is suggested as a plausible reason for such exercise limitation.
Cardiovascular response
At rest, compared to healthy non-smokers, narghile smokers had significant increases in HR (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), SBP (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), and DBP (Koubaa, Triki, Trabelsi, et al., 2015a). These results are in line with the previously published data evaluating the effects of narghile use on resting cardiovascular data (Al-Kubati et al., 2006; Alomari et al., 2014; Ben Saad et al., 2014; Bentur et al., 2014; El-Zaatari et al., 2015; Pratiti & Mukherjee, 2019; Shaikh et al., 2008). The abovementioned effects were previously discussed in the subsection related to the chronic effects of narghile use on 6MWT data.
At peak exercise, compared to healthy non-smokers, narghile smokers had a difficulty in maintaining appropriate cardiac output (i.e., lower HR and higher HR reserve [Hawari et al., 2017]). Narghile use seems to modify the chronotropic response to exercise, and therefore it may induce chronotropic incompetence. These results, similar to those observed in cigarette smokers (Lauer et al., 1997; Papathanasiou et al., 2013), can be explained by a downregulation of beta-adrenergic receptors (Lauer et al., 1997).
At recovery, one study reported a lower recovery index in narghile smokers than in healthy non-smokers and cigarette smokers (Koubaa, Triki, Trabelsi, et al., 2015a)(Table 2). The recovery index is directly related to HR decline after exercise (Koubaa, Triki, Trabelsi, et al., 2015a). First, the latter is a useful marker of cardiac autonomic control, mediated by the vagal reactivation and the sympathetic decline (Morise, 2004; Pecanha et al., 2017). Second, there is a direct relation between HR achieved at peak exercise and the subsequent decline during recovery (Leclerc, 2017). This may partially explain why narghile smokers had a blunted recovery index than healthy non-smokers. Third, attenuated recovery index is indicative of attenuated HR recovery, which is a marker of cardiac limitation of exercise capacity (Leclerc, 2017). Fourth, HR recovery has been related to higher risk of CVDs, and it is an independent predictor of mortality (Morshedi-Meibodi et al., 2002; Myers et al., 2007; Qiu et al., 2017; Shetler et al., 2001; Sipila et al., 2019). Finally, both attenuated HR at peak exercise and reduced HR recovery were independently associated with cardiovascular mortality (Myers et al., 2007). These findings highlight the important role for the early detection of the harmful effect of narghile use on health by exploring cardiovascular response during and after a stressful situation, such as CPET.
Ventilatory and muscular responses
Hawari et al. (2017) suggested that “abnormal” ventilatory responses are less likely to explain the reduced exercise capacity, since differences in breathing reserve, E and anaerobic threshold were insignificant between exclusive narghile smokers and healthy non-smokers.
How Can the Harmful Effects of Narghile Use on Animals’ Cardiovascular Data be Explained?
To the best of the authors’ knowledge, no previous experimental study has treated the issue related to the chronic effects of narghile use on animals’ cardiovascular response during exercise. The main results of some experimental studies aiming to determine the harmful effects of narghile use on animals’ resting cardiovascular data and their underlying mechanisms are highlighted in the online Appendix. Briefly, the advanced underlying mechanisms were the following: systemic inflammation, heart inflammation, heart oxidative stress, prothrombotic and thrombotic phenomena, hypercoagulability, apoptosis, genetic changes, gas exchange abnormalities and changes in arterial blood gases, heart morphologic changes, myocardial injury, and cardiotoxicity (Fahim et al., 2014; Nemmar et al., 2015, 2018, 2019, 2020; Nemmar, Al-Salam, Beegam, et al., 2017; Nemmar, Al-Salam, Yuvaraju, et al., 2017; Nemmar, Raza, et al., 2013; Nemmar, Yuvaraju, et al., 2013)
Discussion of the Methodology
Discussion related to the studies’ characteristics and applied exercise tests (i.e., 6MWT vs. CPET) is highlighted in the online Appendix. The subsequent sentences will discuss the following points: smoking data, participants’ characteristics, and some gaps related to the main collected data. Box 2 summarizes some recommendations for designing future studies.
Box 2.
Some recommendations for designing future studies related to the chronic effects of narghile use on cardiovascular response during exercise.
| Issue | Recommendation: authors are encouraged to: |
|---|---|
| Study protocol/design | Blind the investigators/technicians in studies including two
groups or more Design participants from a general population in order to have representative measurements (i.e., avoid convenience sampling) Clearly define the term “healthy” in studies including healthy participants Report as non-inclusion/exclusion criteria, the contraindications of the applied exercise tests |
| Smoking data | Specify the “exclusive” character of narghile use, and to
include only exclusive narghile smokers Use the local applied terms to refer to narghile use (e.g., “shisha” or “narghile” for studies performed in North Africa) Specify the type of narghile tobacco smoked: tabamel, tombak, Jurak Apply the most used ways to quantify narghile use (e.g., narghile-years) Specify the exact level of exposure to narghile tobacco Specify the time of last narghile use |
| Participant data | Specify the participants’ exact age |
| Sample size | Calculate the sample size |
| Exercise data expression | Express heart rate as a percentage of the predicted maximal
heart rate Express the maximal oxygen uptake in absolute value and as a percentage of the predicted value |
| Exercise influencing factors | Report some morphological data known to influence exercise capacity (e.g., lower limb length, quadriceps strength) |
| Biological data | Explore some biological data (e.g., oxidative stress, inflammation, anemia) |
| Functional respiratory data | Report some functional respiratory data (e.g., lung volumes, diffusion capacity of the alveolar-capillary membrane, blood gas) |
| Some static cardiac data | Explore some echocardiography data (e.g., right ventricular function) |
| Muscle activity data | Explore some markers of secondary myopathy (e.g., blood creatine phosphokinase, electromyogram, muscle biopsy data) |
Smoking Data
Six points related to the smoking data need to be highlighted. First, three studies omitted to specify the “exclusive” character of narghile use (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a). This is a serious lacuna, since the body keeps memory of the smokers’ physiological and behavioral practices (Chaouachi, 2009). In future studies, only exclusive narghile smokers should be included in the group of narghile smokers. Second, different names were used to refer to narghile use (Table 1). Certainly, the name of the tobacco mode depends on the region and the country (Aslam et al., 2014), but the use of terms like “hookah” in Tunisia (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a) or “waterpipe” in Jordan (Hawari et al., 2017) is not realistic. In fact, in Tunisia and/or Jordan, the terms “shisha” and “narghile” are the most popular (Ben Saad, 2015; Chaouachi, 2007; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a). Third, four studies (Hawari et al., 2017; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a) did not mention the type of narghile tobacco smoked. The lack of specification of the tobacco type is a source of confusion. In fact, there are three different forms of narghile tobacco (tabamel, tombak and jurak), and the pattern differs between tombak and jurak, in comparison to tabamel (Ben Saad, 2009; Chaouachi, 2009; Nemmar et al., 2020). In the future, the used type of narghile tobacco should be noted to allow comparisons between studies. Fourth, the quantification ways of narghile use was different between the retained studies. In the absence of a specific international codification, narghile use quantification in terms of narghile-years (or hookah-years) is the preferred method (Ben Saad et al., 2014; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a). The use of different methods to quantify narghile consumption (hookah-day [Koubaa, Triki, Trabelsi, Baati, et al., 2015], heads-week [Hawari et al., 2017], kg of cumulated tobacco [Ben Saad et al., 2014; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a]) makes comparisons between studies difficult. A specific international codification is needed (Ben Saad, 2009). Fifth, the levels of exposure to narghile tobacco were different between the retained studies (Ben Saad et al., 2014; Hawari et al., 2017; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a). They were imprecise in three studies (>5 hookah-years) (Ben Saad et al., 2014; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), and large in one study (5–145 narghile-years) (Ben Saad et al., 2014). This situation makes comparison between studies difficult. Finally, information about the last narghile use was lacking in three studies (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a). This information is capital in order to avoid confusion between chronic and acute effects of narghile use (Akl et al., 2010; Bou Fakhreddine et al., 2014; El-Zaatari et al., 2015), even on cardiovascular response at exercise.
Participants’ Characteristics
The lack of sample size calculation, noted in four studies (Hawari et al., 2017; Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, Masmoudi, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a), is a serious statistical flaw. In practice, determining the finest size for a study is a statistically central point to guarantee enough power and to distinguish statistical significance, and constitutes a serious step in the design of research procedure (Kang et al., 2008; Khemiss et al., 2015).
Some Gaps Related to the Main Collected Data
Two studies omitted to express HR as a percentage of PMHR (Koubaa, Triki, Trabelsi, Baati, et al., 2015; Koubaa, Triki, Trabelsi, et al., 2015a). This is a gap as achieving age-predicted values for maximal HR during exercise is often used as a reflection of maximal or near maximal effort (e.g., achieving at least 85% of PMHR is an indicator of sufficient participant effort). Moreover, HR expressed as a percentage of PMHR is more objective than absolute value for comparison between participants.
According to recommendations, O2max should be expressed both in absolute value and as a percentage of the predicted value (Aguilaniu & Wallaert, 2013; ATS/ACCP, 2003). The latter expression mode allows to predict normal achievement of maximal exercise capacity, comparison between participants and determination of the anaerobic threshold normality (e.g., normal range of anaerobic threshold [40%–80%] of O2max predicted) (Aguilaniu & Wallaert, 2013; ATS/ACCP, 2003).
Other factors influencing exercise capacity were not reported and should be studied in the future: (a) morphological data (e.g., lower limb length, quadriceps strength) (Ben Saad et al., 2014; Troosters et al., 1999); (b) biological data (e.g., oxidative stress, inflammation, anemia) (Ben Moussa et al., 2016; Ebner et al., 2016; Rosado-Perez & Mendoza-Nunez, 2018); (c) functional respiratory data (e.g., lung volumes, diffusion capacity of the alveolar-capillary membrane, blood gas) (ATS/ACCP, 2003); (d) echocardiography data (e.g., right ventricular function) (Badagliacca et al., 2017); and (e) myopathy, possible consequence of sedentary lifestyle, oxidative stress, systemic inflammation, or hypoxia (Ben Saad et al., 2014).
Conclusion
This systematic review illustrates the negative effects of narghile use on cardiovascular response to exercise with reduction in both submaximal and maximal exercise capacities. Given the harmful effect of narghile use on cardiovascular health, early detection of its effects on resting cardiovascular data/status and on cardiovascular response to exercise is of great importance. This supports the need for global action to prompt cessation of this smoking habit. Future studies, taking into account the methodological limitations highlighted in this systematic review, are encouraged.
Supplemental Material
Supplemental material, sj-docx-1-jmh-10.1177_1557988321997706 for The Chronic Effects of Narghile Use on Males’ Cardiovascular Response During Exercise: A Systematic Review by Faten Chaieb and Helmi Ben Saad in American Journal of Men's Health
Acknowledgments
Authors wish also to thank Professor Samir Boukattaya for his invaluable contribution in the improvement of the quality of the writing in the present paper.
Footnotes
Author Contributions: FC and HBS: Literature search, data collection, analysis of data, manuscript preparation, and review of manuscript. Both authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HBS reports personal fees from AstraZeneca, Saiph, Teriak, Hikma, Chiesi, and Opalia Recordati. FC declares no conflicts of interest concerning this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Helmi Ben Saad  https://orcid.org/0000-0002-7477-2965
https://orcid.org/0000-0002-7477-2965
Data Availability Statement: Data will be available upon request from the corresponding author: helmi.bensaad@rns.tn
Supplemental Material: Supplemental material for this article is available online.
References
- Aguilaniu B., Wallaert B. (2013). From interpretation of cardiopulmonary exercise testing to medical decision. Revue des maladies respiratoires, 30(6), 498–515. 10.1016/j.rmr.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Akl E. A., Gaddam S., Gunukula S. K., Honeine R., Jaoude P. A., Irani J. (2010). The effects of waterpipe tobacco smoking on health outcomes: a systematic review. International Journal of Epidemiology, 39(3), 834–857. 10.1093/ije/dyq002 [DOI] [PubMed] [Google Scholar]
- Al-Kubati M., Al-Kubati A. S., al’Absi M., Fiser B. (2006). The short-term effect of water-pipe smoking on the baroreflex control of heart rate in normotensives. Autonomic Neuroscience: Basic & Clinical, 126–127, 146–149. 10.1016/j.autneu.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Alomari M. A., Khabour O. F., Alzoubi K. H., Shqair D. M., Eissenberg T. (2014). Central and peripheral cardiovascular changes immediately after waterpipe smoking. Inhalation Toxicology, 26(10), 579–587. 10.3109/08958378.2014.936572 [DOI] [PubMed] [Google Scholar]
- Alomari M. A., Khabour O. F., Alzoubi K. H., Shqair D. M., Stoner L. (2015). Acute vascular effects of waterpipe smoking: Importance of physical activity and fitness status. Atherosclerosis, 240(2), 472–476. 10.1016/j.atherosclerosis.2015.02.047 [DOI] [PubMed] [Google Scholar]
- Al Suwaidi J., Zubaid M., El-Menyar A. A., Singh R., Asaad N., Sulaiman K., Al Mahmeed W., Al-Shereiqi S., Akbar M., Al Binali H. A. (2012). Prevalence and outcome of cigarette and waterpipe smoking among patients with acute coronary syndrome in six Middle-Eastern countries. European Journal of Preventive Cardiology, 19(1), 118–125. 10.1177/1741826710393992 [DOI] [PubMed] [Google Scholar]
- Aslam H. M., Saleem S., German S., Qureshi W. A. (2014). Harmful effects of shisha: Literature review. International archives of medicine, 7, 16. 10.1186/1755-7682-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATS. (2002). A. T. S. Committee on proficiency standards for clinical pulmonary function laboratories. ATS statement: Guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine, 166(1), 111–117. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- ATS/ACCP. (2003). American thoracic, society, American college of chest, physicians. ATS/ACCP statement on cardiopulmonary exercise testing. American Journal of Respiratory and Critical Care Medicine, 167(2), 211–277. 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- Azar R. R., Frangieh A. H., Mroue J., Bassila L., Kasty M., Hage G., Kadri Z. (2016). Acute effects of waterpipe smoking on blood pressure and heart rate: A real-life trial. Inhalation Toxicology, 28(8), 339–342. 10.3109/08958378.2016.1171934 [DOI] [PubMed] [Google Scholar]
- Badagliacca R., Papa S., Valli G., Pezzuto B., Poscia R., Reali M., Manzi G., Giannetta E., Berardi D., Sciomer S., Palange P., Fedele F., Naeije R., Vizza C. D. (2017). Right ventricular dyssynchrony and exercise capacity in idiopathic pulmonary arterial hypertension. The European Respiratory Journal, 49(6). 10.1183/13993003.01419-2016 [DOI] [PubMed] [Google Scholar]
- Ben Moussa S., Rouatbi S., Ben Saad H. (2016). Incapacity, handicap, and oxidative stress markers of male smokers with and without COPD. Respiratory Care, 61(5), 668–679. 10.4187/respcare.04420 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Burbank A. D. (2016). Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends in Cardiovascular Medicine, 26(6), 515–523. 10.1016/j.tcm.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Saad H. (2009). The narghile and its effects on health. Part I: The narghile, general description and properties. Revue de pneumologie clinique, 65(6), 369–375. 10.1016/j.pneumo.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Ben Saad H. (2010). The narghile and its effects on health. Part II: The effects of the narghile on health. Revue de pneumologie clinique, 66(2), 132–144. 10.1016/j.pneumo.2009.08.011 (Le narguile et ses effets sur la sante. Partie II : les effets du narguile sur la sante). [DOI] [PubMed] [Google Scholar]
- Ben Saad H. (2015). Methodological problems in the article comparing lung function profiles and aerobic capacity of adult cigarette and hookah smokers after 12 weeks intermittent training. The Libyan Journal of Medicine, 10(1), 27760. 10.3402/ljm.v10.27760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Saad H., Babba M., Boukamcha R., Ghannouchi I., Latiri I., Mezghenni S., Zedini C., Rouatbi S. (2014). Investigation of exclusive narghile smokers: Deficiency and incapacity measured by spirometry and 6-minute walk test. Respiratory Care, 59(11), 1696–1709. 10.4187/respcare.03058 [DOI] [PubMed] [Google Scholar]
- Ben Saad H., Khemis M., Bougmiza I., Prefaut C., Aouina H., Mrizek N., Garrouche A., Zbidi A., Tabka Z. (2011). Spirometric profile of narghile smokers. Revue des maladies respiratoires, 28(7), e39–e51. 10.1016/j.rmr.2008.10.001 [DOI] [PubMed] [Google Scholar]
- Ben Saad H., Prefaut C., Tabka Z., Mtir A. H., Chemit M., Hassaoune R., Ben Abid T., Zara K., Mercier G., Zbidi A., Hayot M. (2009). 6-minute walk distance in healthy North Africans older than 40 years: Influence of parity. Respiratory Medicine, 103(1), 74–84. 10.1016/j.rmed.2008.07.023 [DOI] [PubMed] [Google Scholar]
- Bentur L., Hellou E., Goldbart A., Pillar G., Monovich E., Salameh M., Scherb I., Bentur Y. (2014). Laboratory and clinical acute effects of active and passive indoor group water-pipe (narghile) smoking. Chest, 145(4), 803–809. 10.1378/chest.13-0960 [DOI] [PubMed] [Google Scholar]
- Bhatnagar A., Maziak W., Eissenberg T., Ward K. D., Thurston G., King B. A., Sutfin E. L., Cobb C. O., Griffiths M., Goldstein L. B., Rezk-Hanna M. (2019). Water pipe (Hookah) smoking and cardiovascular disease risk: A scientific statement from the American heart association. Circulation, 139(19), e917–e936. 10.1161/CIR.0000000000000671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. A. (1982). Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise, 14(5), 377–381. https://www.ncbi.nlm.nih.gov/pubmed/7154893 [PubMed] [Google Scholar]
- Bou Fakhreddine H. M., Kanj A. N., Kanj N. A. (2014). The growing epidemic of water pipe smoking: Health effects and future needs. Respiratory Medicine, 108(9), 1241–1253. 10.1016/j.rmed.2014.07.014 [DOI] [PubMed] [Google Scholar]
- Chaouachi K. (2009). Hookah (shisha, narghile) smoking and Environmental Tobacco Smoke (ETS). A critical review of the relevant literature and the public health consequences. International Journal of Environmental Research and Public Health, 6(2), 798–843. 10.3390/ijerph6020798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouachi K. T. (2007). The narghile (hookah, shisha, goza) epidemic and the need for clearing up confusion and solving problems related with model building of social situations. The Scientific World Journal, 7, 1691–1696. 10.1100/tsw.2007.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetta A., Zanini A., Pisi G., Aiello M., Tzani P., Neri M., Olivieri D. (2006). Reference values for the 6-min walk test in healthy subjects 20–50 years old. Respiratory Medicine, 100(9), 1573–1578. 10.1016/j.rmed.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Cobb C. O., Sahmarani K., Eissenberg T., Shihadeh A. (2012). Acute toxicant exposure and cardiac autonomic dysfunction from smoking a single narghile waterpipe with tobacco and with a “healthy” tobacco-free alternative. Toxicology Letters, 215(1), 70–75. 10.1016/j.toxlet.2012.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner N., Jankowska E. A., Ponikowski P., Lainscak M., Elsner S., Sliziuk V., Steinbeck L., Kube J., Bekfani T., Scherbakov N., Valentova M., Sandek A., Doehner W., Springer J., Anker S. D., von Haehling S. (2016). The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the studies investigating co-morbidities aggravating heart failure. International Journal of Cardiology, 205, 6–12. 10.1016/j.ijcard.2015.11.178 [DOI] [PubMed] [Google Scholar]
- El-Zaatari Z. M., Chami H. A., Zaatari G. S. (2015). Health effects associated with waterpipe smoking. Tobacco Control, 24 Suppl 1, i31–i43. 10.1136/tobaccocontrol-2014-051908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim M. A., Nemmar A., Al-Salam S., Dhanasekaran S., Shafiullah M., Yasin J., Hassan M. Y. (2014). Thromboembolic injury and systemic toxicity induced by nicotine in mice. General Physiology and Biophysics, 33(3), 345–355. 10.4149/gpb_2014012 [DOI] [PubMed] [Google Scholar]
- Hawari F. I., Obeidat N. A., Ayub H., Ghonimat I., Eissenberg T., Dawahrah S., Beano H. (2013). The acute effects of waterpipe smoking on lung function and exercise capacity in a pilot study of healthy participants. Inhalation Toxicology, 25(9), 492–497. 10.3109/08958378.2013.806613 [DOI] [PubMed] [Google Scholar]
- Hawari F. I., Obeidat N. A., Ghonimat I. M., Ayub H. S., Dawahreh S. S. (2017). The effect of habitual waterpipe tobacco smoking on pulmonary function and exercise capacity in young healthy males: A pilot study. Respiratory Medicine, 122, 71–75. 10.1016/j.rmed.2016.11.024 [DOI] [PubMed] [Google Scholar]
- Hayano J., Yamada M., Sakakibara Y., Fujinami T., Yokoyama K., Watanabe Y., Takata K. (1990). Short- and long-term effects of cigarette smoking on heart rate variability. The American Journal of Cardiology, 65(1), 84–88. 10.1016/0002-9149(90)90030-5 [DOI] [PubMed] [Google Scholar]
- Hering D., Kucharska W., Kara T., Somers V. K., Narkiewicz K. (2010). Smoking is associated with chronic sympathetic activation in hypertension. Blood Pressure, 19(3), 152–155. 10.3109/08037051.2010.484150 [DOI] [PubMed] [Google Scholar]
- Kammoun R., Ben Saad H. (2020). From deficiency to handicap in the respiratory field: Lung function tests (LFT) norms and quality of life (QOL) questionnaires validated for the Tunisian population. La Tunisie medicale, 98(5), 378–395. https://www.ncbi.nlm.nih.gov/pubmed/32548841 [PubMed] [Google Scholar]
- Kang M., Ragan B. G., Park J. H. (2008). Issues in outcomes research: An overview of randomization techniques for clinical trials. Journal of Athletic Training, 43(2), 215–221. 10.4085/1062-6050-43.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khemiss M., Rouatbi S., Berrezouga L., Ben Saad H. (2015). Critical analysis of the published literature about the effects of narghile use on oral health. The Libyan Journal of Medicine, 10, 30001. 10.3402/ljm.v10.30001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinos P., Manolis A., Pittaras A., Doumas M., Giannelou A., Panagiotakos D. B., Faselis C., Narayan P., Singh S., Myers J. (2009). Exercise capacity and mortality in hypertensive men with and without additional risk factors. Hypertension (Dallas, Tex: 1979), 53(3), 494–499. 10.1161/HYPERTENSIONAHA.108.127027 [DOI] [PubMed] [Google Scholar]
- Koubaa A., Elloumi A., Trabelsi H., Masmoudi L., Sahnoun Z., Hakim A. (2019). Physical activity improves cardiovascular capacity and prevents decline in lung function caused by smoking: Efficacy of the intermittent and continuous training program. Science & Sports, 34(2), e101–e108. 10.1016/j.scispo.2018.08.006 [DOI] [Google Scholar]
- Koubaa A., Triki M., Trabelsi H., Baati H., Sahnoun Z., Hakim A. (2015). The effect of a 12-week moderate intensity interval training program on the antioxidant defense capability and lipid profile in men smoking cigarettes or hookah: a cohort study. The Scientific World Journal, 2015, 639369. 10.1155/2015/639369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubaa A., Triki M., Trabelsi H., Masmoudi L., Sahnoun Z., Hakim A. (2015). Changes in antioxidant defense capability and lipid profile after 12-week low- intensity continuous training in both cigarette and hookah smokers: A follow-up study. PLoS One, 10(6), e0130563. 10.1371/journal.pone.0130563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubaa A., Triki M., Trabelsi H., Masmoudi L., Zeghal K. N., Sahnoun Z., Hakim A. (2015. a). Effect of low-intensity continuous training on lung function and cardiorespiratory fitness in both cigarette and hookah smokers. African Health Sciences, 15(4), 1170–1181. 10.4314/ahs.v15i4.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubaa A., Triki M., Trabelsi H., Masmoudi L., Zeghal K. N., Sahnoun Z., Hakim A. (2015. b). Lung function profiles and aerobic capacity of adult cigarette and hookah smokers after 12 weeks intermittent training. The Libyan Journal of Medicine, 10(1), 26680. 10.3402/ljm.v10.26680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer M. S., Pashkow F. J., Larson M. G., Levy D. (1997). Association of cigarette smoking with chronotropic incompetence and prognosis in the Framingham Heart Study. Circulation, 96(3), 897–903. 10.1161/01.cir.96.3.897 [DOI] [PubMed] [Google Scholar]
- Leclerc K. (2017). Cardiopulmonary exercise testing: A contemporary and versatile clinical tool. Cleveland Clinic Journal of Medicine, 84(2), 161–168. 10.3949/ccjm.84a.15013 [DOI] [PubMed] [Google Scholar]
- Lucini D., Bertocchi F., Malliani A., Pagani M. (1996). A controlled study of the autonomic changes produced by habitual cigarette smoking in healthy subjects. Cardiovascular Research, 31(4), 633–639. https://www.ncbi.nlm.nih.gov/pubmed/8689656 [PubMed] [Google Scholar]
- Mahler D. A., Mackowiak J. I. (1995). Evaluation of the short-form 36-item questionnaire to measure health-related quality of life in patients with COPD. Chest, 107(6), 1585–1589. 10.1378/chest.107.6.1585 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., Group P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morise A. P. (2004). Heart rate recovery: Predictor of risk today and target of therapy tomorrow? Circulation, 110(18), 2778–2780. 10.1161/01.CIR.0000147615.62634.48 [DOI] [PubMed] [Google Scholar]
- Morshedi-Meibodi A., Larson M. G., Levy D., O’Donnell C. J., Vasan R. S. (2002). Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study). The American Journal of Cardiology, 90(8), 848–852. 10.1016/s0002-9149(02)02706-6 [DOI] [PubMed] [Google Scholar]
- Muller P. T., Barbosa G. W., O’Donnell D. E., Neder J. A. (2019). Cardiopulmonary and muscular interactions: Potential implications for exercise (in)tolerance in symptomatic smokers without chronic obstructive pulmonary disease. Frontiers in Physiology, 10, 859. 10.3389/fphys.2019.00859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Tan S. Y., Abella J., Aleti V., Froelicher V. F. (2007). Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. European Journal of Cardiovascular Prevention and Rehabilitation, 14(2), 215–221. 10.1097/HJR.0b013e328088cb92 [DOI] [PubMed] [Google Scholar]
- Nelson M. D., Rezk-Hanna M., Rader F., Mason O. R., Tang X., Shidban S., Rosenberry R., Benowitz N. L., Tashkin D. P., Elashoff R. M., Lindner J. R., Victor R. G. (2016). Acute effect of hookah smoking on the human coronary microcirculation. The American Journal of Cardiology, 117(11), 1747–1754. 10.1016/j.amjcard.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A., Al-Salam S., Beegam S., Yuvaraju P., Ali B. H. (2019). Gum Arabic ameliorates impaired coagulation and cardiotoxicity induced by water-pipe smoke exposure in mice. Frontiers in Physiology, 10, 53. 10.3389/fphys.2019.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A., Al-Salam S., Beegam S., Yuvaraju P., Oulhaj A., Ali B. H. (2017). Water-pipe smoke exposure-induced circulatory disturbances in mice, and the influence of betaine supplementation thereon. Cellular Physiology and Biochemistry, 41(3), 1098–1112. 10.1159/000464117 [DOI] [PubMed] [Google Scholar]
- Nemmar A., Al-Salam S., Beegam S., Yuvaraju P., Zaaba N. E., Yasin J., Ali B. H. (2020). Waterpipe tobacco smoke inhalation triggers thrombogenicity, cardiac inflammation and oxidative stress in mice: Effects of flavouring. International Journal of Molecular Sciences, 21(4). 10.3390/ijms21041291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A., Al-Salam S., Yuvaraju P., Beegam S., Ali B. H. (2018). Exercise training mitigates water pipe smoke exposure-induced pulmonary impairment via inhibiting NF-kappaB and activating Nrf2 signalling pathways. Oxidative Medicine and Cellular Longevity, 2018, 7459612. 10.1155/2018/7459612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A., Al-Salam S., Yuvaraju P., Beegam S., Yasin J., Ali B. H. (2017). Chronic exposure to water-pipe smoke induces cardiovascular dysfunction in mice. American Journal of Physiology. Heart and Circulatory Physiology, 312(2), H329–H339. 10.1152/ajpheart.00450.2016 [DOI] [PubMed] [Google Scholar]
- Nemmar A., Raza H., Yuvaraju P., Beegam S., John A., Yasin J., Hameed R. S., Adeghate E., Ali B. H. (2013). Nose-only water-pipe smoking effects on airway resistance, inflammation, and oxidative stress in mice. Journal of Applied Physiology, 115(9), 1316–1323. 10.1152/japplphysiol.00194.2013 [DOI] [PubMed] [Google Scholar]
- Nemmar A., Yuvaraju P., Beegam S., Ali B. H. (2015). Short-term nose-only water-pipe (shisha) smoking exposure accelerates coagulation and causes cardiac inflammation and oxidative stress in mice. Cellular Physiology and Biochemistry, 35(2), 829–840. 10.1159/000369741 [DOI] [PubMed] [Google Scholar]
- Nemmar A., Yuvaraju P., Beegam S., John A., Raza H., Ali B. H. (2013). Cardiovascular effects of nose-only water-pipe smoking exposure in mice. American Journal of Physiology. Heart and Circulatory Physiology, 305(5), H740–H746. 10.1152/ajpheart.00200.2013 [DOI] [PubMed] [Google Scholar]
- Papathanasiou G., Georgakopoulos D., Papageorgiou E., Zerva E., Michalis L., Kalfakakou V., Evangelou A. (2013). Effects of smoking on heart rate at rest and during exercise, and on heart rate recovery, in young adults. Hellenic Journal of Cardiology, 54(3), 168–177. https://www.ncbi.nlm.nih.gov/pubmed/23685653 [PubMed] [Google Scholar]
- Pecanha T., Bartels R., Brito L. C., Paula-Ribeiro M., Oliveira R. S., Goldberger J. J. (2017). Methods of assessment of the post-exercise cardiac autonomic recovery: A methodological review. International Journal of Cardiology, 227, 795–802. 10.1016/j.ijcard.2016.10.057 [DOI] [PubMed] [Google Scholar]
- Pratiti R., Mukherjee D. (2019). Epidemiology and adverse consequences of hookah/waterpipe use: A systematic review. Cardiovascular & Hematological Agents in Medicinal Chemistry, 17(2), 82–93. 10.2174/1871525717666190904151856 [DOI] [PubMed] [Google Scholar]
- Qasim H., Alarabi A. B., Alzoubi K. H., Karim Z. A., Alshbool F. Z., Khasawneh F. T. (2019). The effects of hookah/waterpipe smoking on general health and the cardiovascular system. Environmental Health and Preventive Medicine, 24(1), 58. 10.1186/s12199-019-0811-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S., Cai X., Sun Z., Li L., Zuegel M., Steinacker J. M., Schumann U. (2017). Heart rate recovery and risk of cardiovascular events and all-cause mortality: A meta-analysis of prospective cohort studies. Journal of the American Heart Association, 6(5). 10.1161/JAHA.117.005505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezk-Hanna M., Benowitz N. L. (2019). Cardiovascular effects of hookah smoking: Potential implications for cardiovascular risk. Nicotine & Tobacco Research, 21(9), 1151–1161. 10.1093/ntr/nty065 [DOI] [PubMed] [Google Scholar]
- Rosado-Perez J., Mendoza-Nunez V. M. (2018). Relationship between aerobic capacity with oxidative stress and inflammation biomarkers in the blood of older Mexican urban-dwelling population. Dose Response, 16(2), 1559325818773000. 10.1177/1559325818773000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostagno C., Gensini G. F. (2008). Six minute walk test: A simple and useful test to evaluate functional capacity in patients with heart failure. Internal and Emergency Medicine, 3(3), 205–212. 10.1007/s11739-008-0130-6 [DOI] [PubMed] [Google Scholar]
- Selim G. M., Fouad H., Ezzat S. (2013). Impact of shisha smoking on the extent of coronary artery disease in patients referred for coronary angiography. Anadolu kardiyoloji dergisi, 13(7), 647–654. 10.5152/akd.2013.191 [DOI] [PubMed] [Google Scholar]
- Sergysels R., Hayot M. (1997). Evaluation of exercise-induced dyspnea. Revue de pneumologie clinique, 53(5), 278–282. https://www.ncbi.nlm.nih.gov/pubmed/9616842 (Evaluation de la dyspnee a l’effort.) [PubMed] [Google Scholar]
- Shafique K., Mirza S. S., Mughal M. K., Arain Z. I., Khan N. A., Tareen M. F., Ahmad I. (2012). Water-pipe smoking and metabolic syndrome: a population-based study. PLoS One, 7(7), e39734. 10.1371/journal.pone.0039734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh R. B., Vijayaraghavan N., Sulaiman A. S., Kazi S., Shafi M. S. (2008). The acute effects of waterpipe smoking on the cardiovascular and respiratory systems. Journal of Preventive Medicine and Hygiene, 49(3), 101–107. https://www.ncbi.nlm.nih.gov/pubmed/19278135 [PubMed] [Google Scholar]
- Shetler K., Marcus R., Froelicher V. F., Vora S., Kalisetti D., Prakash M., Do D., Myers J. (2001). Heart rate recovery: Validation and methodologic issues. Journal of the American College of Cardiology, 38(7), 1980–1987. 10.1016/s0735-1097(01)01652-7 [DOI] [PubMed] [Google Scholar]
- Sibai A. M., Tohme R. A., Almedawar M. M., Itani T., Yassine S. I., Nohra E. A., Isma’eel H. A. (2014). Lifetime cumulative exposure to waterpipe smoking is associated with coronary artery disease. Atherosclerosis, 234(2), 454–460. 10.1016/j.atherosclerosis.2014.03.036 [DOI] [PubMed] [Google Scholar]
- Sipila K., Tikkakoski A., Alanko S., Haarala A., Hernesniemi J., Lyytikainen L. P., Viik J., Lehtimaki T., Nieminen T., Nikus K., Kahonen M. (2019). Combination of low blood pressure response, low exercise capacity and slow heart rate recovery during an exercise test significantly increases mortality risk. Annals of Medicine, 51(7–8), 390–396. 10.1080/07853890.2019.1684550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghizadeh N., Vonk J. M., Boezen H. M. (2016). Lifetime smoking history and cause-specific mortality in a cohort study with 43 years of follow-up. PLoS One, 11(4), e0153310. 10.1371/journal.pone.0153310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troosters T., Gosselink R., Decramer M. (1999). Six minute walking distance in healthy elderly subjects. The European Respiratory Journal, 14(2), 270–274. 10.1034/j.1399-3003.1999.14b06.x [DOI] [PubMed] [Google Scholar]
- Voorrips L. E., Ravelli A. C., Dongelmans P. C., Deurenberg P., Van Staveren W. A. (1991). A physical activity questionnaire for the elderly. Medicine and Science in Sports and Exercise, 23(8), 974-979. https://www.ncbi.nlm.nih.gov/pubmed/1956274 [PubMed] [Google Scholar]
- Waziry R., Jawad M., Ballout R. A., Al Akel M., Akl E. A. (2017). The effects of waterpipe tobacco smoking on health outcomes: an updated systematic review and meta-analysis. International Journal of Epidemiology, 46(1), 32–43. 10.1093/ije/dyw021 [DOI] [PubMed] [Google Scholar]
- WHO. (2015). World Health Organisation Advisory note: Waterpipe tobacco smoking: 2nd edition health effects, research needs and recommended actions for regulators. Retrieved December 30, 2020, from http://www.who.int/tobacco/publications/prod_regulation/waterpipesecondedition/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jmh-10.1177_1557988321997706 for The Chronic Effects of Narghile Use on Males’ Cardiovascular Response During Exercise: A Systematic Review by Faten Chaieb and Helmi Ben Saad in American Journal of Men's Health