Abstract
BACKGROUND AND PURPOSE: Subarachnoid hemorrhage (SAH) constitutes an important neurologic emergency. Some authors have suggested that fluid-attenuated inversion recovery (FLAIR) MR imaging can detect SAH that may not be apparent on CT scans but may be revealed by lumbar puncture. We sought to determine how often FLAIR MR imaging findings are positive for SAH in cases with negative CT findings and positive lumbar puncture results.
METHODS: The CT scans and FLAIR MR images of all patients with suspected SAH during a 3-year interval (2000–2002) were retrospectively reviewed by a blinded reader. Among these cases, we identified 12 with CT findings that were negative for SAH, lumbar puncture results that were positive for SAH, and FLAIR MR imaging findings that were available for review. Eleven of the 12 patients had undergone FLAIR MR imaging within 2 days of CT and lumbar puncture. The 12 patients with negative CT findings were comprised of six male and six female patients with an age range of 7 to 69 years. We evaluated the true and false negative and positive FLAIR MR imaging findings for SAH by using the lumbar puncture results as the gold standard. The FLAIR MR imaging findings of 12 additional patients without SAH (as revealed by lumbar puncture) were used as control data for a blinded reading.
RESULTS: For all 12 control cases without SAH, the FLAIR MR imaging findings were interpreted correctly. Of the 12 cases that had positive lumbar puncture results but false-negative CT findings for SAH, FLAIR MR imaging findings were true-positive in only two cases and were false-negative in 10. One of the two true-positive cases had the highest concentration of RBC in the series (365 k/cc), and the other had the second highest value of RBC (65 k/cc).
CONCLUSION: FLAIR MR imaging cannot replace lumbar puncture to detect the presence of SAH. FLAIR MR imaging findings are infrequently positive (16.7%) when CT findings are negative for SAH. This is likely because there is a minimum concentration of RBC/cc that must be exceeded for CSF to become hyperintense on FLAIR MR images.
During the past several years, a number of investigators have suggested that fluid-attenuated inversion recovery (FLAIR) MR imaging is the most sensitive MR imaging technique for detection of subarachnoid disease. A number of studies showing the ability of FLAIR MR imaging to reveal subarachnoid hemorrhage (SAH), meningitis, chemical irritation of the meninges, and subarachnoid seeding of neoplasm have been published in the radiologic literature (1–7). Many examples have been cited in which FLAIR MR imaging was more sensitive than CT for detection of subarachnoid diseases such as chemical meningitis, inflammatory meningitis, and neoplastic infiltration of the subarachnoid space where the CT attenuation difference often is inapparent (6, 8–11). While the notion that FLAIR MR imaging would be more sensitive than CT for SAH (with which the globin in the blood causes the detectable change in attenuation) may seem counterintuitive, it has nonetheless been repeatedly found to be true in the literature (3, 5, 7, 12–14).
For this study, we accepted the premise that FLAIR MR imaging is superior to CT for the detection of SAH, based on the literature cited above. This then begged the question of how FLAIR MR imaging compares with the gold standard, lumbar puncture. We sought to determine whether FLAIR MR imaging could be useful in the setting of cases of acute SAH confirmed by lumbar puncture that had CT findings negative for SAH. In this select group of cases, we also sought to determine whether a specific concentration of RBC within the subarachnoid space could be determined for which FLAIR MR imaging would be sensitive. We hypothesized that FLAIR MR imaging would detect most of the CT-negative cases of SAH but that there would be a threshold of blood concentration that would have to be exceeded, measured in the thousands of RBC/cc.
Methods
The CT reports of all patients who underwent arteriography for the evaluation of suspected acute SAH during a 3-year interval (2000–2002) were retrospectively reviewed. We selected only those cases in which the CT findings were negative for SAH. We cross-referenced this list by using the electronic patient records of patients who had undergone FLAIR MR imaging and lumbar puncture. Twelve patients had negative CT findings, SAH revealed by lumbar puncture, and FLAIR MR imaging findings available. Of the 12, 11 had undergone MR imaging within 2 days of lumbar puncture and CT. The remaining patient underwent MR imaging 7 days after lumbar puncture and CT. The sample group of 12 patients consisted of six male and six female patients ranging in age from 7 to 69 years. The FLAIR MR images were blindly reviewed by an experienced neuroradiologist (D.M.Y.), and the results were compared with the official reports of those MR images in the electronic patient records. To balance the sample group and provide a control group, FLAIR MR images of 12 patients without SAH, as revealed by lumbar puncture, were randomly interspersed among the patients with negative CT findings and positive lumbar puncture results for SAH. The blinded reader could not determine whether a patient did or did not have positive lumbar puncture results based on the FLAIR MR images being reviewed.
For the FLAIR MR imaging, the following parameters were used: 8800/170 (TR/TE); inversion time, 2200 ms; number of excitations, 2; matrix, 256 × 192 exposed to zero-filled interpolation to 512 × 512. Tailored RF pulses were used, as were spatial presaturation pulses for superiorly oriented flow. Imaging was performed on a General Electric (Milwaukee, WI) LX imaging unit using high speed or echo-speed gradients. Only the FLAIR MR images were presented to the reader for interpretation. The reader scored the studies based on a simple positive or negative basis for SAH or intraventricular hemorrhage.
The lumbar puncture results were analyzed for number of RBC/cc. If more than one tube was collected, the values for the all the tubes were recorded for number of RBC (Table 1). In all cases, the clinicians thought that the clinical picture and the lumbar puncture results suggested SAH.
TABLE:
Patient data with documented LP showing SAH
| Patient #, age, sex | FLAIR | RBC/cc |
Angio/Dx | CT/LP-MR interval | |||
|---|---|---|---|---|---|---|---|
| CSF1 | CSF2 | CSF3 | CSF4 | ||||
| 1, 69M | − | 37 | 119 | Normal/trauma | 2d | ||
| 2, 56F | − | 69 | 100 | 1680 | 344 | Aneurysm | 1d |
| 3, 44F | − | 80 | 6225 | Aneurysm | 0d | ||
| 4, 57M | − | 700 | 711 | 404 | Fibromuscular dysplasia/migraine | 1d | |
| 5, 33M | − | 1634 | 1623 | Normal/sickle cell crisis | 0d | ||
| 6, 45M | − | 2249 | 2129 | Dissection/dissecting aneurysm | 2d | ||
| 7, 7F | − | 2932 | Normal/migraine | 0d | |||
| 8, 52F | − | 3550 | 2550 | Aneurysm | 1d | ||
| 9, 39F | − | 29710 | 33700 | Aneurysm | 2d | ||
| 10, 56M | − | 33525 | 36425 | 14750 | 10675 | Normal/unknown | 0d |
| 11, 51M | − | 120149 | Normal/trauma/stroke | 5d | |||
| 12, 58F | + | 65363 | 68750 | 72450 | 40950 | Normal/lupus | 2d |
| 13, 60M | + | 364875 | 414750 | Normal/unknown | 7d | ||
Cases for which the blinded reviewer’s analysis for the presence of SAH differed from the official MR imaging report in the electronic patient record interpreted by a neuroradiologist were settled by the interpretation of a third neuroradiologist (B.Y.) who also was blinded to the status of the patient. This occurred in one instance.
Results
We defined lumbar puncture results as positive if there was evidence of blood in the CSF that did not completely clear by the repeat and/or final test tube was shown and if the clinicians, in consideration of the clinical symptoms and the lumbar puncture results, thought arteriography was warranted. In our study, the RBC/cc number of RBC in positive CSF samples ranged from 37 to 364,875 RBC/cc in the first test tube and from 344 to 414,750 RBC/cc in the last tube collected (Table 1).
Of these 12 cases with CT findings that were negative for SAH, 10 had false-negative FLAIR MR imaging studies (Fig 1) and two had true-positive FLAIR MR imaging findings (Fig 2). The RBC/cc counts were 364,875 and 65,363 in the first CSF tubes collected and 414,750 and 40,950 in the fourth tubes, respectively. These were the two highest values for the first tube and the two highest values for the last tubes. FLAIR MR imaging findings were false-negative in 10 of 12 cases and were negative for all studies in which the first tube RBC/cc counts were <65,000. Of the 12 cases with positive lumbar puncture results, five had aneurysms documented as the source of the SAH, one had head trauma as the cause, two had migraine headaches, and four had miscellaneous or unknown causes of the SAH. In the 12 control cases in which no SAH was revealed by lumbar puncture, the FLAIR MR imaging findings were uniformly negative.
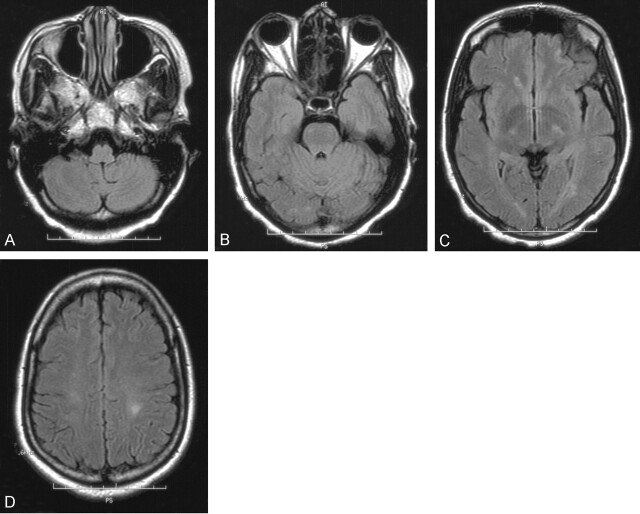
Fig 1.
Case 9, with negative CT and negative FLAIR MR imaging findings. Although lumbar puncture results were positive, FLAIR MR images obtained at multiple levels were negative for SAH.
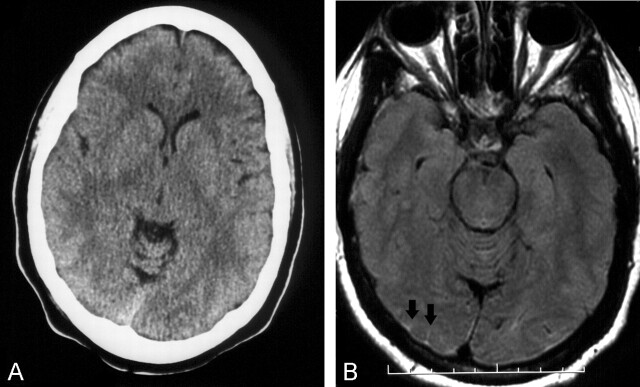
Fig 2.
Case 12, with negative CT and positive FLAIR MR imaging findings.
A, No SAH shown on CT scan.
B, FLAIR MR image interpreted as showing SAH based on presence of high intensity in right occipital lobe sulci (arrows).
Discussion
In most instances, lumbar puncture is the most valuable technique for determining whether a patient has experienced SAH. Unfortunately, in some cases in which evidence of significant edema or mass effect is present, lumbar puncture cannot be performed because of the dangers of potentially life-threatening herniation of intracranial structures. In other cases, diffuse swelling of the brain may mask (or simulate) the presence of SAH, which, because of the swelling, cannot be otherwise evaluated with lumbar puncture.
Having a technique that is significantly more sensitive to the presence of acute SAH than is CT and that is analogous in sensitivity to lumbar puncture would be valuable to the neuroradiologic field. FLAIR MR imaging has been purported to be this panacea. FLAIR MR imaging also offers the potential of diagnosing non-acute SAH. It is well known that CT is less sensitive to the presence of subacute and chronic SAH at a time when xanthochromia may be the only finding revealed by lumbar puncture because of the lysis of RBC (15, 16). Because 90% of extravasated blood is cleared from the CSF within 1 week, only half of all cases of SAH remain detectable on CT scans at 7 days (17). In 1995, van der Wee et al (18) reported that CT findings may be normal in ≤5% of the cases that are investigated within 1 or 2 days after SAH. In this retrospective study, we included all the FLAIR MR images that were obtained within 2 days of the CT and lumbar puncture studies, except for one case (case 12) in which the FLAIR MR imaging was performed 1 week after CT and lumbar puncture. We included that case because the FLAIR MR images showed the SAH both by blinded review and by the initial prospective reporting. It is possible that this patient may have rebled during the 7-day interval between the lumbar puncture and the FLAIR MR imaging.
The FLAIR MR imaging sequence produces heavily T2-weighted images with nulling of the signal intensity of CSF by using an inversion time that usually ranges from 1800 to 2500 ms and a long TE. Because of the suppressed signal intensity of bulk water on FLAIR MR images, the conspicuity of lesions located in areas adjacent to or filled with CSF, such as the periventricular zones, increases. However, if the CSF has alterations in its T1 or T2 values, its signal intensity may not suppress. This phenomenon in cases of SAH may be caused by the T2 prolongation or T1 shortening effects of higher protein concentrations of bloody CSF (3, 7). T1 will shorten with increasing hematocrit.
To assess how sensitive FLAIR MR imaging is to the presence of RBC in the subarachnoid space, Noguchi et al (7) conducted phantom studies simulating SAH of varying concentrations ranging from 0% blood (100% water) to 100% blood (with a hematocrit of 45%) by volume. They performed CT and FLAIR MR imaging of the test tube phantoms to assess the concentrations needed to detect SAH for the two modalities. As the hematocrit increased, the attenuation on CT scans and the signal intensity on FLAIR MR images also increased. At a hematocrit of 27% blood, the mixture was denser on CT scans than was the normal cortex and therefore was detectable. Above a hematocrit of 22.4% blood, the mixture was more hyperintense than the normal cortex on FLAIR MR images. By increasing the TE of the FLAIR MR imaging (from a baseline of 119) and thereby increasing its T2 weighting, the authors were able to increase the sensitivity of the FLAIR MR imaging sequence to the presence of hemorrhage. Using a TE of 160, they found that blood with a hematocrit of 9% was hyperintense to cortex. They concluded that FLAIR MR imaging was far more sensitive than CT in the detection of acute SAH diluted by CSF, particularly if the pulse sequence parameters were optimized (7). The relatively high CT threshold (hematocrit of 27%) accounts for cases of SAH with negative CT findings.
Our data indicated that FLAIR MR imaging findings were infrequently positive (16.7%) when CT findings were negative for SAH and that there was a lower limit criterion for RBC/cc below which FLAIR MR imaging did not detect SAH. In this study, this level was approximately 65 k/cc for the first tube.
Woodcock et al (5) injected different volumes of autologous arterial blood into the basal cisterns of rabbits to compare the sensitivity of CT and FLAIR MR imaging to the presence of SAH. The sensitivity of FLAIR MR imaging to SAH was 89% (16 of 18 rabbits), whereas that of CT was 39% (seven of 18 rabbits) in that model. The confidence in detecting the SAH was also much higher with FLAIR MR imaging than with CT. The specificity for both CT and FLAIR MR imaging was 100%.
Pitfalls Associated with FLAIR MR Imaging
Dechambre et al (19) noted that false-positive FLAIR MR imaging findings for SAH could occur for patients who had strokes and who had previously undergone contrast-enhanced perfusion studies. They noted that the disruption of the blood-brain barrier may result in leakage of protein or contrast material chelates or both into the subarachnoid space. Contrast material leaching into the CSF has been observed to occur in other patients undergoing high dose contrast-enhanced MR angiographic studies, in patients who are in renal failure, and in patients with active seizures who are receiving contrast agents. Examples in which high protein may be the source of the high intensity on FLAIR MR images include cases of sinus thromboses and case of neurosarcoidosis (8, 20).
Other false-positive findings occur when the oxygen tension in the CSF is elevated from the use of inspired oxygen fractions >0.60 during general anesthesia (21). Although propofol administration (22) was initially implicated, the overriding impact of the paramagnetic influences of dissolved oxygen in the CSF leading to T1 shortening have been subsequently validated (23).
On FLAIR MR images, high signal intensity from the CSF may also result from artifacts (24). Ghosting artifacts are caused by inflow of non-nulled CSF into a section with a high CSF flow rate and are seen mainly in the basal cisterns (24, 25). They are unusual over the convexities. These artifacts are reduced with non-section-selective inversion pulses.
In our study, 12 patients with documented SAH, as revealed by lumbar puncture, were selected retrospectively from the angiography records. Although FLAIR MR imaging detected SAH in two cases in which it was missed by CT, the remaining 10 cases had false-negative FLAIR MR imaging findings. The cases that had negative FLAIR MR imaging findings, in general, had lower concentrations of hemorrhage than did the two cases that had positive FLAIR MR imaging findings. We recognized a lower threshold of 65,000 RBC/cc that led to both negative CT findings and negative FLAIR MR imaging findings.
Although FLAIR MR imaging seems to be superior to CT for detecting SAH, MR imaging often is impractical to perform acutely in the emergency department because emergent MR facilities are less readily available than are CT scanners. Often, unstable or irritable patients do not readily/easily undergo MR imaging. In cases in which the patient is more stable, MR imaging may be superior to CT in detecting blood in the CSF because the attenuation changes shown on CT scans resolve more rapidly than do the changes shown on FLAIR MR images, as previously reported (14, 26). This makes MR imaging a valuable method for identifying hemorrhage in patients with negative CT findings and positive lumbar puncture results who are not referred until 1 or 2 weeks after symptom onset (27).
Despite the high sensitivity of FLAIR MR imaging, this study suggests that lumbar puncture is still an indispensable step in the exclusion of SAH in patients with convincing histories and negative CT findings. van Gijn and Rinkel (28) suggest that lumbar puncture is best performed between 6 and 12 hr after onset of headache so that RBC lysis can occur, thereby yielding xanthochromia. The presence of xanthochromia is an excellent means of distinguishing a “bloody tap” (which would not have time to produce xanthochromia) from a sample that contains blood secondary to SAH (15, 16, 18, 29, 30). The hemoglobin pigments cause the CSF to have a yellow tinge after centrifugation and are invariably detectable for at least 2 weeks after initial bleed (15, 16). In our study, lumbar puncture was performed within 1 or 2 days of CT in 11 of 12 cases.
The weaknesses in this study include small sample size, failure to scan the entire CNS axis to exclude a spinal source of SAH, delays between lumbar puncture and FLAIR MR imaging, and failure to conclusively define the cause of SAH in a number of cases. When compiling a sample of cases of SAH with negative CT findings and reducing the sample to include only those with arteriography results and FLAIR MR imaging findings obtained within 48 hr of each other, very few cases remain to be studied. The cases of SAH with negative CT findings and negative arteriography results (six of our cases) often have unusual (or unknown) diagnoses. Those that include spinal MR imaging to search for sources of SAH are even fewer, especially if one imparts a time limit on accumulating patients. We think that the RBC would not have cleared from the CSF within 48 hr based on the report presented by van Gijn and Donger (17). We are not in any way advocating replacing lumbar puncture with FLAIR MR imaging, especially considering the results of this analysis. It is standard practice, however, that before performing lumbar puncture in a person with the worst headache of one’s life that an imaging study is performed to exclude mass effect, which could induce herniation from the lumbar puncture. In cases in which one has ready access to an emergency department MR imaging unit and CT scanner, before performing lumbar puncture, one may be tempted to use FLAIR MR imaging as the survey for SAH. Or, considering negative CT findings for SAH in that same scenario and considering risk factors associated with lumbar puncture, such as cerebral edema, mass effect, recent lumbar spine surgery, anticoagulant use, and coagulopathy, MR imaging may be considered as the next step. We think our results show that FLAIR MR imaging is not necessarily the panacea that we might have hoped. The added “pick-up rate” after negative CT findings is limited, and the gold standard of lumbar puncture remains untarnished.
Conclusion
In a selected group of 12 patients with SAH revealed by lumbar puncture and negative CT findings, FLAIR MR imaging findings were positive in only two cases. These two cases had the two highest concentrations of RBC/cc shown by lumbar puncture results. Our data suggest that FLAIR MR imaging findings were infrequently positive when CT findings were negative for SAH and that a lower limit criterion exists for RBC/cc below which FLAIR MR imaging will not detect SAH. This suggests that despite claims of remarkable sensitivity of FLAIR MR imaging for SAH, lumbar puncture is still an indispensable tool in the work-up of a patient for SAH.
References
- 1.Noguchi K, Ogawa T, Inugami A, Toyoshima H, Okudera T, Uemura K. MR of acute subarachnoid hemorrhage: a preliminary report of fluid-attenuated inversion-recovery pulse sequences. AJNR Am J Neuroradiol 1994;15:1940–1943 [PMC free article] [PubMed] [Google Scholar]
- 2.Noguchi K, Ogawa T, Inugami A, et al. Acute subarachnoid hemorrhage: MR imaging with fluid-attenuated inversion recovery pulse sequences. Radiology 1995;196:773–777 [DOI] [PubMed] [Google Scholar]
- 3.Bakshi R, Kamran S, Kinkel PR, et al. Fluid-attenuated inversion-recovery MR imaging in acute and subacute cerebral intraventricular hemorrhage. AJNR Am J Neuroradiol 1999;20:629–636 [PMC free article] [PubMed] [Google Scholar]
- 4.Kuker W, Thiex R, Rohde I, Rohde V, Thron A. Experimental acute intracerebral hemorrhage: value of MR sequences for a safe diagnosis at 1.5 and 0.5 T. Acta Radiol 2000;41:544–552 [DOI] [PubMed] [Google Scholar]
- 5.Woodcock RJ Jr, Short J, Do HM, Jensen ME, Kallmes DF. Imaging of acute subarachnoid hemorrhage with a fluid-attenuated inversion recovery sequence in an animal model: comparison with non-contrast-enhanced CT. AJNR Am J Neuroradiol 2001;22:1698–1703 [PMC free article] [PubMed] [Google Scholar]
- 6.Singh SK, Leeds NE, Ginsberg LE. MR imaging of leptomeningeal metastases: comparison of three sequences. AJNR Am J Neuroradiol 2002;23:817–821 [PMC free article] [PubMed] [Google Scholar]
- 7.Noguchi K, Seto H, Kamisaki Y, Tomizawa G, Toyoshima S, Watanabe N. Comparison of fluid-attenuated inversion-recovery MR imaging with CT in a simulated model of acute subarachnoid hemorrhage. AJNR Am J Neuroradiol 2000;21:923–927 [PMC free article] [PubMed] [Google Scholar]
- 8.Bode MK, Tikkakoski T, Tuisku S, Kronqvist E, Tuominen H. Isolated neurosarcoidosis: MR findings and pathologic correlation. Acta Radiol 2001;42:563–567 [DOI] [PubMed] [Google Scholar]
- 9.Kuwahara S, Kawada M, Uga S. Cryptococcal meningoencephalitis presenting with an unusual magnetic resonance imaging appearance: case report. Neurol Med Chir (Tokyo) 2001;41:517–521 [DOI] [PubMed] [Google Scholar]
- 10.Maeda M, Yagishita A, Yamamoto T, Sakuma H, Takeda K. Abnormal hyperintensity within the subarachnoid space evaluated by fluid-attenuated inversion-recovery MR imaging: a spectrum of central nervous system diseases. Eur Radiol 2003. Apr 24 [Epub ahead of print] [DOI] [PubMed]
- 11.Singer MB, Atlas SW, Drayer BP. Subarachnoid space disease: diagnosis with fluid-attenuated inversion-recovery MR imaging and comparison with gadolinium-enhanced spin-echo MR imaging: blinded reader study. Radiology 1998;208:417–422 [DOI] [PubMed] [Google Scholar]
- 12.Wiesmann M, Mayer TE, Yousry I, Medele R, Hamann GF, Bruckmann H. Detection of hyperacute subarachnoid hemorrhage of the brain by using magnetic resonance imaging. J Neurosurg 2002;96:684–689 [DOI] [PubMed] [Google Scholar]
- 13.Mitchell P, Wilkinson ID, Hoggard N, et al. Detection of subarachnoid haemorrhage with magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2001;70:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noguchi K, Ogawa T, Seto H, et al. Subacute and chronic subarachnoid hemorrhage: diagnosis with fluid-attenuated inversion-recovery MR imaging. Radiology 1997;203:257–262 [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen M, Hasan D, Blijenberg BG, Hijdra A, van Gijn J. Xanthochromia after subarachnoid haemorrhage needs no revisitation. J Neurol Neurosurg Psychiatry 1989;52:826–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Gijn J, Vermeulen M, Hasan D. Xanthochromia. Lancet 1989;2:1036. [DOI] [PubMed] [Google Scholar]
- 17.van Gijn J, van Dongen KJ. The time course of aneurysmal hemorrhage on computed tomograms. Neuroradiology 1982;23:153–156 [DOI] [PubMed] [Google Scholar]
- 18.van der Wee N, Rinkel GJ, Hasan D, van Gijn J. Detection of subarachnoid haemorrhage on early CT: is lumbar puncture still needed after a negative scan? J Neurol Neurosurg Psychiatry 1995;58:357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dechambre SD, Duprez T, Grandin CB, Lecouvet FE, Peeters A, Cosnard G. High signal in cerebrospinal fluid mimicking subarachnoid haemorrhage on FLAIR following acute stroke and intravenous contrast medium. Neuroradiology 2000;42:608–611 [DOI] [PubMed] [Google Scholar]
- 20.Bozzao A, Bastianello S, Bozzao L. Superior sagittal sinus thrombosis with high-signal-intensity CSF mimicking subarachnoid hemorrhage on MR FLAIR images. AJR Am J Roentgenol 1997;169:1183–1184 [DOI] [PubMed] [Google Scholar]
- 21.Frigon C, Jardine DS, Weinberger E, Heckbert SR, Shaw DW. Fraction of inspired oxygen in relation to cerebrospinal fluid hyperintensity on FLAIR MR imaging of the brain in children and young adults undergoing anesthesia. AJR Am J Roentgenol 2002;179:791–796 [DOI] [PubMed] [Google Scholar]
- 22.Filippi CG, Ulug AM, Lin D, Heier LA, Zimmerman RD. Hyperintense signal abnormality in subarachnoid spaces and basal cisterns on MR images of children anesthetized with propofol: new fluid-attenuated inversion recovery finding. AJNR Am J Neuroradiol 2001;22:394–399 [PMC free article] [PubMed] [Google Scholar]
- 23.Deliganis AV, Fisher DJ, Lam AM, Maravilla KR. Cerebrospinal fluid signal intensity increase on FLAIR MR images in patients under general anesthesia: the role of supplemental O2. Radiology 2001;218:152–156 [DOI] [PubMed] [Google Scholar]
- 24.Wu HM, Yousem DM, Chung HW, Guo WY, Chang CY, Chen CY. Influence of imaging parameters on high-intensity cerebrospinal fluid artifacts in fast-FLAIR MR imaging. AJNR Am J Neuroradiol 2002;23:393–399 [PMC free article] [PubMed] [Google Scholar]
- 25.Herlihy AH, Hajnal JV, Curati WL, et al. Reduction of CSF and blood flow artifacts on FLAIR images of the brain with k-space reordered by inversion time at each slice position (KRISP). AJNR Am J Neuroradiol 2001;22:896–904 [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa T, Inugami A, Fujita H, et al. MR diagnosis of subacute and chronic subarachnoid hemorrhage: comparison with CT. AJR Am J Roentgenol 1995;165:1257–1262 [DOI] [PubMed] [Google Scholar]
- 27.Renowden SA, Molyneux AJ, Anslow P, Byrne JV. The value of MRI in angiogram-negative intracranial haemorrhage. Neuroradiology 1994;36:422–425 [DOI] [PubMed] [Google Scholar]
- 28.van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001;124:249–278 [DOI] [PubMed] [Google Scholar]
- 29.Vermeulen M, van Gijn J. The diagnosis of subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1990;53:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Gijn J. Slip-ups in diagnosis of subarachnoid haemorrhage. Lancet 1997;349:1492. [DOI] [PubMed] [Google Scholar]