Abstract
BACKGROUND AND PURPOSE: A potential source of complication at carotid endarterectomy (CEA) is cerebral ischemia caused by hypoperfusion during clamping of the carotid artery. Configuration of primary collateral pathways may be a major risk factor for development of transient cerebral ischemia during clamping. We investigated whether circle of Willis morphology on 3D time-of-flight (TOF) MR angiograms can reliably predict transient ischemia during vascular clamping of the internal carotid artery (ICA) in patients undergoing CEA.
METHODS: Three-dimensional TOF MR angiography and CEA were performed in 117 patients. Patients had unilateral stenosis (n = 86), unilateral stenosis with contralateral occlusion (n = 21), or bilateral severe stenosis (n = 10) of the ICA. Circle of Willis morphology on preoperative 3D TOF MR angiograms was analyzed and correlated with intraoperative history of ischemia during vascular clamping of the ICA.
RESULTS: Patients with unilateral stenosis or bilateral severe stenosis experienced transient ischemia during clamping of ICA at a low rate (11 [11%] of 96). In these groups, we found no specific circle of Willis patterns associated with development of intraoperative ischemia. However, patients with contralateral ICA occlusion experienced ischemia frequently during clamping of the ICA (12 [57%] of 21). In this group, incompleteness of the circle of Willis was significantly related to the risk of intraoperative ischemia (P = .005).
CONCLUSION: In patients without contralateral ICA occlusion, circle of Willis morphology on 3D TOF MR angiograms cannot predict the development of intraoperative ischemia. However, in patients with contralateral ICA occlusion, incompleteness of the posterior part of the circle of Willis is a significant risk factor for development of ischemia during vascular clamping of the ICA.
Carotid endarterectomy (CEA) is an important treatment for patients with more severe stenosis of the carotid artery (1–3). But one of the potential sources of procedure-related complication is cerebral ischemia caused by cerebral hypoperfusion during clamping of the carotid artery to perform CEA (1, 3–6). About 10–20% of patients undergoing CEA will not tolerate cross-clamping of the internal carotid artery (ICA) and will require placement of an intraluminal shunt to prevent intraoperative cerebral ischemia (3). However, the use of an intraluminal shunt during CEA remains controversial. It may reduce cerebral hypoperfusion but may also carry an additional risk of complications such as thromboembolism or dissection of the carotid artery (7). When shunt surgery is performed selectively, there are several methods to assess the need for shunt placement. In most cases, patients are monitored intraoperatively through continuous electroencephalography, neurologic evaluation of the awake patient under local anesthesia, or carotid stump pressure measurements (1).
In patients with carotid artery stenosis, cerebral perfusion is dependent not only on the degree of stenosis, but also on the collateral pathways (3, 4, 8–12). Several collateral pathways may contribute to cerebral perfusion. Primary collateral vessels are the circle of Willis, which can respond quickly to low perfusion pressure with simple reversal of flow. Secondary collateral vessels such as ophthalmic artery and pial arteries require time for recruitment and are usually acquired in response to the stress of chronic hypoperfusion (8–10). Therefore, the configuration of primary collateral pathways may be a major risk factor for development of transient cerebral ischemia during clamping of the ICA. In the literature, conventional angiography, transcranial Doppler ultrasonography, and phase contrast MR angiography were used in several reports to evaluate whether collateral cerebral blood flow through the anterior and posterior communicating arteries protects against cerebral ischemia during CEA (3, 4, 12, 13). Three-dimensional time-of-flight (TOF) MR angiography is a noninvasive and sensitive technique for detecting the anatomy of the circle of Willis, which can provide much important information without the need for invasive study (14).
In this study, we investigated whether circle of Willis morphology on 3D TOF MR angiograms can reliably predict transient ischemia during vascular clamping of the ICA in patients underoing CEA.
Methods
Patient Population
Among 231 patients who had ICA stenosis and were treated with CEA at our institution from 1997 to 2001, we recruited 117 patients who had available preoperative 3D TOF intracranial MR angiograms. Our institutional review board approved the study protocol before initiating the review. There were 105 male and 12 female patients with a mean age of 66 years (range, 46–82 years). Patients were divided into three groups: unilateral ICA stenosis (group A, n = 86), unilateral ICA stenosis with contralateral ICA occlusion (group B, n = 21), and bilateral severe stenosis (group C, n = 10). The patients were categorized into three groups because we hypothesized that contralateral ICA occlusion or bilateral severe ICA stenosis could be associated with a higher risk of ischemia during clamping of a carotid artery than could unilateral ICA stenosis alone.
MR Angiography
Preoperative MR angiography was performed with two 1.5-T MR imaging systems (Magnetom Vision; Siemens, Erlangen, Germany and Signa CVi; GE Medical System, Milwaukee, WI). In all patients, 3D TOF MR angiography of the circle of Willis and contrast material–enhanced MR angiography of cervical arteries were performed. We obtained 3D multislab TOF MR angiograms from the petrous portion of the ICA to the level of the genu portion of the corpus callosum by using the following imaging parameters: 25∼35/3∼7/1 (TR/TE/excitation), flip angle of 20°, five slabs, effective section thickness of 0.8 mm, field of view of 200 mm, and matrix of 256 × 256. The angiographic images were reconstructed with a maximum intensity projection (MIP) algorithm. Two sets of 15 MIP images were obtained by rotation of the stacked images along the vertical axis and horizontal axis. We also performed contrast-enhanced MR angiography of proximal cervical vessels from the aortic arch to the level of central skull base by using an intravenous bolus injection of 20 mL (3–4 mL/s) of gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany) and the following imaging parameters: 3∼7/1∼2/1 [TR/TE/NEX], flip angle of 40°, single coronal slab thickness of 6.4–8 cm, effective section thickness of 1–2 mm, field of view of 260–280 mm, and matrix of 256 × 256. The angiographic images of proximal cervical arteries were reconstructed with the MIP algorithm. One set of 15 MIP images was obtained by rotation of the stacked images along a vertical axis. Percentage stenosis of the ICA was determined from contrast-enhanced MR angiograms per North American Symptomatic Carotid Endarterectomy Trial criteria. Those with less than 30% stenosis were graded as mild, 30–69% as moderate, and 70–99% as severe (3). Posterior circulations were normal in all subjects.
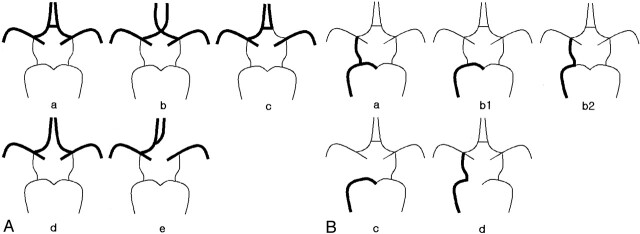
Two independent radiologists (J.H.L., C.G.C.) evaluated circle of Willis morphology on preoperative 3D TOF MR angiograms. The features of anterior and posterior parts of the circle of Willis were evaluated separately and categorized into five patterns (Fig 1). According to the vessel size seen on 3D TOF MR angiograms, the component vessels consisting of circle of Willis were regarded as normal, hypoplastic, or absent. In this study, we considered the anterior communicating artery as normally patent if the segment of anterior communicating artery was clearly visualized or junctions of A1 and A2 segments were in close contact and therefore were not separable with each other on 3D TOF MR angiograms. Hypoplastic A1 segment of anterior cerebral artery was defined when the diameter of the artery was less than half of the contralateral A1 segment. In a case of posterior communicating artery or P1 segment of posterior cerebral artery, hypoplasia was defined when the diameter of the artery was less than half of contralateral P1 segment or posterior communicating artery, respectively. If one of the components of anterior or posterior circle of Willis was hypoplastic, it was considered as indeterminate for its role as a collateral pathway of cerebral blood flow. If one of the components of the circle of Willis was absent, it was considered as incomplete. After review of 3D TOF MR angiograms, circle of Willis morphology was classified as good communicator when at least one of the two parts was complete; it was considered as poor communicator when either part was incomplete. If the circle of Willis morphology took such pattern as indeterminate-indeterminate, indeterminate-incomplete, or incomplete-indeterminate, it was considered as uncertain for its role as a collateral and was excluded from the final analysis.
Fig 1.
Schematic views of anatomic variations of the anterior (A) and posterior (B) parts of the circle of Willis. Only the right side is presented. The left side is a mirror image of the right side.
A, Demonstrable anterior communicating artery (a), approximated both proximal A1 segments without demonstrable anterior communicating artery (b), hypoplastic contralateral A1 segment (c), widely separated both A1 segments with no demonstrable anterior communicating artery (d), absence of contralateral A1 segment (e). The anterior part of the circle of Willis is considered as complete in types a and b, incomplete in types d and e, or indeterminate in type c.
B, Demonstrable ipsilateral posterior communicating artery and P1 segment (a), hypoplastic ipsilateral posterior communicating artery or ipsilateral P1 segment (b1 and b2), no demonstrable ipsilateral posterior communicating artery (c), no demonstrable ipsilateral P1 segment (d). The posterior part of the circle of Willis is considered as complete in type a, incomplete in types c and d, or indeterminate in type b (b1 or b2).
Carotid Endarterectomy
Experienced vascular surgeons (D.K.K., G.E.K.) performed CEA with the patient under local anesthesia. All patients were given a local blockade of the cervical plexus to monitor transient cerebral ischemia in an alert state. After clamping of the carotid artery, patients were monitored whether transient cerebral ischemia such as mentality change or decreased hand motor power occurred. In those patients with symptoms of cerebral ischemia, a temporary intraluminal shunt was introduced and then, CEA was performed. In all other patients, CEA was performed without an intraluminal shunt. An episode of intraoperative hypotension was not observed in any patient.
Statistical Analysis
The completeness of the anterior or posterior part of the circle of Willis was correlated with the intraoperative history of transient ischemia during carotid clamping in each patient group. Statistical significance was evaluated with the Pearson χ2 test. P values less than .05 were considered to indicate a statistically significant difference. Interobserver variability in the interpretation of circle of Willis morphology on 3D TOF MR angiograms was calculated by using the Cohen κ statistic. Agreement was classified as moderate (κ > 0.40–0.79), excellent (κ > 0.80–0.99), or perfect (κ = 1.00).
Results
Table 1 shows demographic data and preoperative degree of ICA stenosis for the three groups of patients, according to those with and those without transient ischemia during carotid clamping. Compared with patients in group A, those in group B showed a much higher rate of transient ischemia during carotid clamping (12.8% in group A versus 57.1% in group B). None of the patients in group C experienced ischemia during CEA. Table 2 shows the distribution of morphologic patterns of the anterior and posterior parts of the circle of Willis in the three patient groups. In group A, complete pattern of the anterior part of circle of Willis was the most frequent type (69 [80%] of 86), but in the posterior part an incomplete pattern was the most frequent type (64 [74%] of 86). The frequency of complete pattern of the anterior part in group B (nine [43%] of 21) was lower than that of group A, but the frequency of incomplete pattern of posterior part in group B (16 [76%] of 21) was similar to that of group A. There was excellent interobserver agreement for the interpretation of 3D TOF MR angiograms (κ = 0.835). Table 3 shows the status of the entire circle of Willis in the group without contralateral occlusion (groups A and C together) and the group with contralateral occlusion (group B). Statistical analysis revealed that the risk of intraoperative transient ischemia was significantly increased in group B (patients with contralateral ICA occlusion) when the circle of Willis was a poor communicator (P = .005). In the group without contralateral occlusion, we could not find a specific pattern of circle of Willis related to the risk of intraoperative ischemia. Even in eight patients who had incomplete patterns of both anterior and posterior parts of the circle of Willis, only one patient developed ischemia during vascular clamping of the ICA. However, in the group with contralateral occlusion (group B), all 12 patients who developed ischemia during CEA had an incomplete pattern of the posterior part of the circle of Willis. Eight of them also had an incomplete pattern of the anterior part of the circle of Willis (Fig 2), and the other four patients had a complete pattern of the anterior part. Another four patients in group B, who had complete anterior and incomplete posterior parts of the circle of Willis, did not develop intraoperative ischemia (Table 4, Fig 3).
TABLE 1:
Patient demographic data and preoperative degree of ICA stenosis
| Group A, Unilateral Stenosis (n = 86) |
Group B, Contralateral Occlusion (n = 21) |
Group C, Bilateral Severe Stenosis (n = 10) |
||||
|---|---|---|---|---|---|---|
| Ischemia* (n = 11) | No Ischemia* (n = 75) | Ischemia* (n = 12) | No Ischemia* (n = 9) | Ischemia* (n = 0) | No Ischemia* (n = 10) | |
| Demographic data | ||||||
| Mean age (y) | 63.8 | 66.8 | 64.5 | 63.1 | ND | 66.3 |
| Male/Female | 11/0 | 67/8 | 10/2 | 9 | ND | 8/2 |
| Degree of ICA stenosis | ||||||
| Clamped side | ||||||
| Mild to moderate | 0 | 20 | 6 | 1 | ND | 0 |
| Severe | 11 | 55 | 6 | 8 | ND | 10 |
| Contralateral side | ||||||
| Normal | 4 | 55 | 0 | 0 | ND | 0 |
| Mild to moderate | 7 | 20 | 0 | 0 | ND | 0 |
| Severe | 0 | 0 | 0 | 0 | ND | 10 |
| Occlusion | 0 | 0 | 12 | 9 | ND | 0 |
Note.—ND indicates no data.
Intraoperative episode.
TABLE 2:
Morphology of anterior and posterior parts of the circle of Willis
| Patterns of Circle of Willis | Group A, Unilateral Stenosis (n = 86) |
Group B, Contralateral Occlusion (n = 21) |
Group C, Bilateral Severe Stenosis (n = 10) |
|||
|---|---|---|---|---|---|---|
| Ischemia (n = 11) | No Ischemia (n = 75) | Ischemia (n = 12) | No Ischemia (n = 9) | Ischemia (n = 0) | No Ischemia (n = 10) | |
| Anterior part | ||||||
| Complete | 10 | 59 | 4 | 5 | ND | 4 |
| Indeterminate | 0 | 10 | 3 | 4 | ND | 3 |
| Incomplete | 1 | 6 | 5 | 0 | ND | 3 |
| Posterior part | ||||||
| Complete | 2 | 5 | 0 | 4 | ND | 2 |
| Indeterminate | 0 | 15 | 0 | 1 | ND | 1 |
| Incomplete | 9 | 55 | 12 | 4 | ND | 7 |
Note.—ND indicates no data. In the anterior part, complete type corresponds to a and b; indeterminate type, c; and incomplete type, d and e in Fig 1A. In the posterior part, complete type corresponds to a; indeterminate type, b1 and b2; and incomplete type, c and d in Fig 1B.
TABLE 3:
Distribution of circle of Willis status in the patients without contralateral occlusion (groups A + C) and those with contralateral occlusion (group B)
| Circle of Willis Status | Group A + C |
Group B |
||
|---|---|---|---|---|
| Ischemia (n = 11) | No Ischemia (n = 85) | Ischemia (n = 12) | No Ischemia (n = 9) | |
| Good communicator | 9 | 68 | 4 | 8 |
| Poor communicator | 2 | 6 | 8 | |
| Uncertain communicator* | 11 | 1 | ||
These are composed of circle of Willis patterns of indeterminate-indeterminate, indeterminate-incomplete, and incomplete-indeterminate.
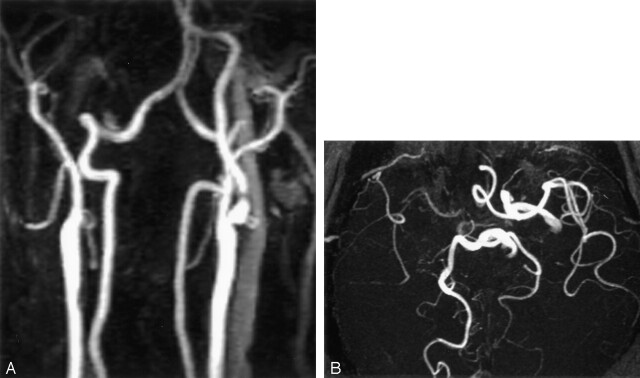
Fig 2.
A 62-year-old man with a history of sudden loss of consciousness immediately after vascular clamping during CEA.
A, Contrast-enhanced MR angiogram shows focal severe stenosis of the left proximal ICA and occlusion of the contralateral ICA.
B, Three-dimensional TOF MR angiogram demonstrates incomplete anterior and posterior patterns of the circle of Willis with absence of the right A1 segment of the anterior cerebral artery and the left posterior communicating artery.
TABLE 4:
Distribution of circle of Willis patterns in patients with contralateral occlusion (group B)
| Anterior | Posterior |
||
|---|---|---|---|
| Incomplete | Indeterminate | Complete | |
| Ischemia (n = 12) | |||
| Incomplete | 8 | 0 | 0 |
| Complete | 4 | 0 | 0 |
| No ischemia (n = 9) | |||
| Indeterminate | 0 | 1 | 3 |
| Complete | 4 | 0 | 1 |
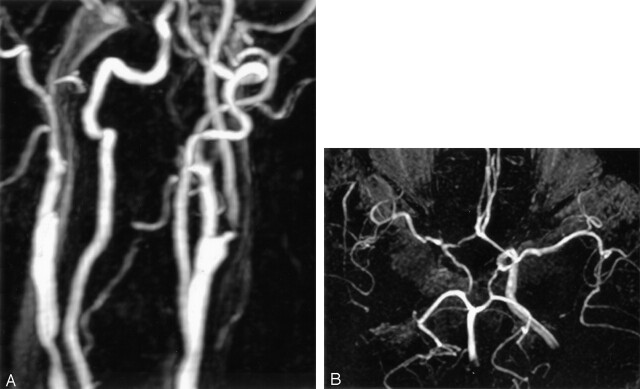
Fig 3.
A 65-year-old man without any neurologic symptoms during CEA.
A, Contrast-enhanced MR angiogram shows short segmental severe stenosis of the left proximal ICA. The contralateral ICA is occluded at the level of the carotid bifurcation.
B, Three-dimensional TOF MR angiogram shows intact both A1 segments, anterior communicating artery, and ipsilateral posterior communicating artery. The circle of Willis is complete in both anterior and posterior parts.
Discussion
The present study relates circle of Willis morphology on preoperative 3D TOF MR angiograms to transient ischemia during the clamping of ICA in CEA procedures. We found that an incomplete circle of Willis increased the risk of transient cerebral ischemia during carotid clamping in patients with contralateral ICA occlusion. But for patients with unilateral ICA stenosis or bilateral severe stenosis, no statistically significant relation was found between the circle of Willis morphology and the presence of intraoperative cerebral ischemia. Detailed analysis of anterior and posterior parts of the circle of Willis showed that in patients with contralateral ICA occlusion, the absence of collateral flow from the posterior circulation is associated with a high risk of transient cerebral ischemia during carotid artery clamping, indicating that the presence of collateral flow from the posterior circulation may protect against hemodynamic compromise of the brain during carotid clamping. Occlusion of the contralateral ICA has been considered as a risk factor for surgery (4, 15). The increased intraoperative risk in patients with contralateral ICA occlusion is related to the whole dependency on collateral flow from the posterior circulation. Therefore, for patients with contralateral occlusion, it may be important to assess carefully the anatomic configuration or completeness of the circle of Willis before surgery or carotid stent placement procedure to avoid potential ischemic brain damage during the clamping or occlusion of the ICA.
Our observation is in line with that of previous reports (9, 16). Schomer et al (9) used conventional angiography and 3D phase-contrast MR angiography to study 29 patients with ICA occlusion and found that a smaller than 1 mm in diameter or absent ipsilateral posterior communicating artery is a risk factor for ischemic cerebral infarction in patients with ICA occlusion. Hendrikse et al (16) used 3D TOF and 2D phase-contrast MR angiography to study 51 patients with unilateral occlusion of the ICA and found that in patients with unilateral ICA occlusion, the presence of collateral flow via the posterior communicating artery is associated with a low prevalence of border zone infarcts.
In the other patient groups who had unilateral stenosis or bilateral severe stenosis, we could not find a specific pattern of the circle of Willis on 3D TOF MR angiograms related to the development of intraoperative cerebral ischemia during the clamping of the ICA. Although 3D TOF MR angiography has been known to have a high sensitivity and specificity for depicting the anatomy of the circle of Willis, it has some limitations in displaying small collateral channels because of turbulent flow, saturation effect of slow flow or long in-plane flow, or slower velocity of blood adjacent to the wall due to laminar flow (14). In addition, in patients with normal cerebral perfusion in the resting state, balanced blood flow into anterior or posterior communicating arteries may not be sufficient to be seen on MR angiograms, which may decrease the sensitivity of MR angiography further in depicting small collateral channels. MIP images do not display this low signal intensity if it does not exceed the signal intensity of the background. Although the anterior or posterior communicating artery appears as hypoplastic or absent on 3D TOF MR angiograms, it could be due to relatively small or no flow in a patent artery. Those can be applied to patients with extremely severe stenosis of the proximal ICA, also. The presence of a severe stenosis at the carotid bifurcation can diminish the flow-related enhancement in the downstream arterial bed. Poor flow-related enhancement throughout the hemisphere can make patent anterior communicators mimic, apparently, hypoplastic ones. In this study, we had four cases with diminished flow-related enhancement due to severe stenosis of ipsilateral proximal ICA. This phenomenon could partly explain our result of no significant relationship between morphologic pattern of the circle of Willis on 3D TOF MR angiograms and transient ischemia during carotid clamping in patients with unilateral ICA stenosis or bilateral severe ICA stenosis. As an example, we can expect that the incompleteness of both the anterior and posterior parts of the circle of willis on 3D TOF MR angiograms is a risk factor for the development of intraoperative ischemia in patients without contralateral ICA occlusion. Contrary to our expectation, however, seven of eight patients who had incomplete circle of Willis in both anterior and posterior parts did not develop ischemia during carotid clamping in the patient groups without ICA occlusion (groups A and C).
Different from our initial hypothesis, no patients had a history of intraoperative ischemia in group C (bilateral severe stenosis). It is not easy to explain the result because the bilateral severe stenosis group might be more complicated and associated with many factors. Though the number of patients was very small (n = 10), one of the factors that could partly explain it is chronic hypoperfusion state. Chronic hypoperfusion could slowly induce inevitable development of secondary collateral pathways, which were not included in our evaluation. Already established secondary collateral vessels might compensate for a sudden decrease of blood supply to the brain during vascular clamping.
Recently, in patients with carotid stenosis, preoperative evaluation of intra- and extracranial cerebrovascular structures depend more and more on noninvasive vascular imaging modalities such as MR or CT angiography because of improved spatial resolution of those techniques (17, 18). In this study, we used 3D TOF MR angiography for the evaluation of major intracranial vessels around the circle of Willis and 3D contrast-enhanced MR angiography for the evaluation of extracranial carotid and vertebral arteries. Three-dimensional TOF MR angiography is an excellent technique for detecting stenosis of major intracranial vessels, which is one of the perioperative risk factors; however, as mentioned in the above paragraph, it has some limitations for the evaluation of important collateral channels of the circle of Willis. For example, we assumed that the anterior communicating artery existed only if both proximal A1 segments were approximated. But just an approximation cannot guarantee the presence of the anterior communicating artery, and this is one of the limitations of our study. Concerning depiction of the anterior or posterior communicating arteries, CT angiography of the circle of Willis may be better than 3D TOF MR angiography (19). Also, high-resolution contrast-enhanced MR angiography is a promising technique because it can cover from the aortic arch to intracranial vessels above the circle of Willis level with sufficient high spatial resolution comparable to that of 3D TOF MR angiography (20). Further studies may be necessary to verify the possibility that high-resolution contrast-enhanced MR angiography can be used as a one-stop study in patients with carotid stenosis, providing all kinds of information not only about the extracranial cerebrovascular structures, but also about the status of intracranial vessels and the circle of Willis.
Conclusion
Assessment of the circle of Willis with 3D TOF MR angiography is useful for predicting the risk of intraoperative ischemia in a group of patients with contralateral ICA occlusion. In this group, the incompleteness of the circle of Willis is associated with high risk of cerebral ischemia during vascular clamping and necessitates an intraluminal shunt procedure. Carotid angioplasty and stent placement can be an alternative treatment option in this patient group.
Footnotes
Presented as a scientific paper at the 88th annual meeting of the Radiological Society of North America, Chicago, IL, 2002.
References
- 1.Rutgers DR, Blankensteijn JD, Van der Grond J. Preoperative MRA flow quantification in CEA patients: flow differences between patients who develop cerebral ischemia and patients who do not develop cerebral ischemia during cross-clamping of the carotid artery. Stroke 2000;31:3021–3028 [DOI] [PubMed] [Google Scholar]
- 2.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–453 [DOI] [PubMed] [Google Scholar]
- 3.Depippo PS, Ascher E, Scheinman M, Yorkovich W, Hingorani A. The value and limitations of magnetic resonance angiography of the circle of Willis in patients undergoing carotid endarterectomy. Cardiovasc Surg 1999;7:27–32 [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Bresnahan MV, Kearse LA Jr, Yanez P, Young TI. Anterior communicating artery collateral flow protection against ischemic change during carotid endarterectomy. J Neurosurg 1993;79:379–382 [DOI] [PubMed] [Google Scholar]
- 5.Zampella E, Morawetz RB, McDowell HA, et al. The importance of cerebral ischemia during carotid endarterectomy. Neurosurgery 1991;29:727–731 [DOI] [PubMed] [Google Scholar]
- 6.Whittemore AD, Kauffman JL, Kohler TR, Mannick JA. Routine electroencephalographic (EEG) monitoring during carotid endarterectomy. Ann Surg 1983;197:707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redekop G, Ferguson G. Correlation of contralateral stenosis and intraoperative electroencephalogram change with risk of stroke during carotid endarterectomy. Neurosurgery 1992;30:191–194 [DOI] [PubMed] [Google Scholar]
- 8.Kluytmans M, Van der Grond J, Van Everdingen KJ, Klijn CJM, Kappelle LJ, Viergener MA. Cerebral hemodynamics in relation to patterns of collateral flow. Stroke 1999;30:1432–1439 [DOI] [PubMed] [Google Scholar]
- 9.Schomer DF, Marks MP, Steinberg GK, et al. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med 1994;330:1565–1570 [DOI] [PubMed] [Google Scholar]
- 10.Müller M, Schimrigk K. Vasomotor reactivity and pattern of collateral blood flow in severe occlusive carotid artery disease. Stroke 1996;27:296–299 [DOI] [PubMed] [Google Scholar]
- 11.Anzola GP, Gasparotti R, Magoni M, Prandini F. Transcranial Doppler sonography and magnetic resonance angiography in the assessment of collateral hemispheric flow in patients with carotid artery disease. Stroke 1995;26:214–217 [DOI] [PubMed] [Google Scholar]
- 12.Schneider PA, Ringelstein EB, Rossman ME, et al. Importance of cerebral collateral pathways during carotid endarterectomy. Stroke 1988;19:1328–1334 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz RB, Jones KM, LeClercq GT, et al. The value of cerebral angiography in predicting cerebral ischemia during carotid endarterectomy. AJR Am J Roentgenol 1992;159:1057–1061 [DOI] [PubMed] [Google Scholar]
- 14.Stock KW, Wetzel S, Kirsch E, Bongartz G, Steinbrich W, Radue EW. Anatomic evaluation of the circle of Willis: MR angiography versus intraarterial digital subtraction angiography. AJNR Am J Neuroradiol 1996;17:1495–1499 [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips MR, Johnson WC, Scott RM, Vollman RW, Levine H, Nabseth DC. Carotid endarterectomy in the presence of contralateral carotid occlusion: the role of EEG and intraluminal shunting. Arch Surg 1979;114:1232–1239 [DOI] [PubMed] [Google Scholar]
- 16.Hendrikse J, Hartkamp MJ, Hillen B, Mali WPTM, van der Grond J. Collateral ability of the circle of Willis in patients with unilateral internal carotid artery occlusion: border zone infarcts and clinical symptoms. Stroke 2001;32:2768–2773 [DOI] [PubMed] [Google Scholar]
- 17.Randoux B, Marro B, Koskas F, et al. Carotid artery stenosis: prospective comparison of CT, three-dimensional gadolinium-enhanced MR and conventional angiography. Radiology 2001;220:179–185 [DOI] [PubMed] [Google Scholar]
- 18.Wutke R, Lang W, Fellner C, et al. High-resolution, contrast-enhanced magnetic resonance angiography with elliptical centric k-space ordering of supra-aortic arteries compared with selective x-ray angiography. Stroke 2002;33:1522–1529 [DOI] [PubMed] [Google Scholar]
- 19.Velthuis BK, van Leeuwen MS, Witkamp TD, Ramos LM, Berkelbach van der Sprenkel JW, Rinkel GJ. Surgical anatomy of the cerebral arteries in patients with subarachnoid hemorrhage: comparison of computerized tomography angiography and digital subtraction angiography. J Neurosurg 2001;95:206–212 [DOI] [PubMed] [Google Scholar]
- 20.Willinek WA, Gieseke J, Conrad R, et al. Randomly segmented central k-space ordering in high-spatial-resolution contrast-enhanced MR angiography of the supraaortic arteries: initial experience. Radiology 2002;225:583–588 [DOI] [PubMed] [Google Scholar]