Abstract
BACKGROUND AND PURPOSE: Reports in the monolingual literature suggest that the cerebellum has an important role in language processing. The purpose of this study was to determine whether bilingual cerebellar functional MR imaging (fMRI) activation differs during the performance of comparable tasks in subjects’ primary and secondary languages.
METHODS: Eight bilingual, right-handed individuals underwent echo-planar fMRI at 1.5 T. They performed semantic (noun-verb association) and phonological (rhyming) tasks in Spanish (primary language) and English (secondary language). Individual and group functional datasets were analyzed using Statistical Parametric Mapping software (SPM99; P < .001 with a 10-voxel spatial extent threshold) and overlaid on T1-weighted anatomic images normalized to a standard (Montreal Neurologic Institute) space. Analysis of variance was performed on laterality indices derived from voxel counts in cerebellar regions of interest (ROIs). Subtraction of group-averaged normalized results from the combined Spanish tasks from the combined English tasks was also performed within SPM99 (P < .001 activation threshold).
RESULTS: Significantly greater lateralilty indices were noted in the English tasks than in the Spanish tasks (mean Spanish LI, 0.3286; mean English LI, 0.5141 [P = .0143]). Overall, more robust activation was seen in the English tasks than in the Spanish tasks. Areas of significantly greater activation existed in the English tasks as compared with the Spanish tasks; these areas were more prominent in the left cerebellar hemisphere.
CONCLUSION: Although both English and Spanish language tasks demonstrate left cerebellar dominance, English tasks demonstrate greater left hemispheric lateralization.
Functional MR imaging (fMRI) is a relatively new and effective noninvasive method for the study of bilingual language processing. Although many reports in the linguistic and functional imaging literature have addressed the issue of whether distinct cerebral neural networks exist for primary language (L1) and secondary language (L2) acquisition and processing in bilingual adults, few have used fMRI. To date, none has specifically addressed cerebellar language processing in healthy bilingual individuals. fMRI does not depict actual neural networks used in task performance; however, it allows inferences to be made regarding such networks on the basis of changes in regional cerebral blood flow associated with adjacent cortical activation.
Reports in the fMRI, positron emission tomography (PET), and magnetic source imaging literature have described differences in activation topography between L1 and L2 processing in bilingual subjects (1–17). Many of these neuroimaging studies have demonstrated topographic differences in language representation between native and second languages, suggesting that distinct neural networks may be utilized for similar cognitive tasks performed in the different languages. Evidence from the aphasia literature and electrophysiologic studies also suggests that different cerebral neural networks may support the acquisition of languages (18–22). fMRI results suggest that a shared neural network for L1 and L2 might exist in bilingual persons, with varing degrees of activity in the common network for processing in the different languages (16).
None of these studies have specifically explored differences in cerebellar activation between L1 and L2. One reason is that the cerebellum has largely been ignored as a major contributor to language processing, and only recently has it been considered to play a substantial role in neurocognition in general. We now know from anatomic, neurosurgical lesional, and, most recently, functional PET and fMRI neuroimaging studies that numerous interconnections exist between the cerebellum and the supratentorial structures, particularly between the cerebellum and frontal-lobe areas of the language-dominant hemisphere (ie, Broca’s area and supplementary motor area) (23). The lateral cerebellar hemispheres send projections to Brodmann areas 6, 44, and 45 via the nucleus ventralis intermedius and the nucleus ventralis anterior of the thalamus and also reciprocal projections from the prefrontal areas to the lateral cerebellum via the pons and red nucleus (23–26). Therefore, the cerebellum might contribute to language function. The purpose of this study was to determine whether cerebellar activation occurs during language processing and whether language-related cerebellar activation differs between L1 and L2 in Spanish-English bilingual individuals.
Methods
Subjects
The subjects included eight healthy adults (six men and two women) aged 29–41 years. All were primary Spanish speakers, with English as their second language. The subjects were originally from Colombia, Argentina, Nicaragua, Peru, or Puerto Rico and held graduate-level medical or scientific degrees. They started learning English in elementary school after the age of 10 years and received further English instruction during their middle- and high-school years. All had attained adequate proficiency to pass standardized English examinations in their respective countries. Each had an additional 1–5 years of English education beyond high school. All subjects rated their fluency with respect to listening, speaking, reading, and writing English as moderate to high. None spoke additional languages. All were right-handed, as determined by means of the Benton Handedness Questionnaire (27). All participants signed written consent forms approved by our institutional review board.
Tasks
The four language tasks used in this study are described in detail in another publication by our group (28). Four language paradigms were used: 1) an English noun-verb association (semantic) task, 2) a Spanish noun-verb association task, 3) an English rhyming (phonological) task, and 4) a Spanish rhyming (phonological) task. All four were block-design paradigms, with identical lengths of visual stimulus presentation (5 seconds each), identical lengths of activation and control blocks, and identical content of control tasks. Although the actual semantic, phonological, and lexical content of the Spanish and English tasks was different, the structure of the tasks was identical. The Spanish noun-verb task, however, was designed to have content as similar to that of the English noun-verb task as possible, though the content of the phonological tasks were necessarily different because Spanish translations of rhyming English word pairs do not necessarily rhyme. One of the authors (J.M.A.) who is highly proficient in Spanish (L1) and English (L2) developed the Spanish tasks. A Macintosh laptop computer running Psyscope software (29) was used to present the visual stimuli for each task via MR imaging–compatible video goggles (Resonance Technology, Inc., Northridge, CA). The participants wore these goggles while they were inside the bore of the MR imaging machine. Before performing the tasks, all subjects underwent a brief training session with Psyscope inside the machine. The subjects were provided written and verbal instructions and given an opportunity to practice the tasks while the investigators monitored their performance. During the actual task and after the initiation of imaging, all responses were recorded with Psyscope by using a keypad to obtain in-magnet response accuracy data.
For each of the four tasks, 20-second blocks of the experimental task alternated with 20-second blocks of the control task, which was the same for all tasks. For the control task, subjects were shown nonsense line drawings with a + sign in one of the lower corners of the slide. The subjects were instructed to press a button on the left side of keypad if the + sign was in the left corner or a button on the right if the sign was in the right corner. Performance on the control task was monitored. Left and right button presses were balanced for the experimental and control tasks.
English and Spanish Noun-Verb Tasks.—
For both the English and Spanish noun-verb tasks, three words were presented in each stimulus. Two verbs were located on the same horizontal line below a single noun. The subjects were asked to press a button on the right side of a keypad with their right thumb if the noun was associated with the verb on the right. Otherwise, they were to press a button on the left with their left thumb if the noun was associated with the verb on the left.
English and Spanish Phonological Tasks.—
In both the English and Spanish phonological tasks, two words were presented in each visual stimulus. The subjects were instructed to decide whether the pair of words rhymed and to press the right button with their right thumb if they did or the left button with their left thumb if they did not.
Imaging
Images were acquired on a 1.5-T system (Vision; Siemens Medical Systems, Iselin, NJ). A three-dimensional structural T1-weighted MPRAGE (magnetization-prepared rapid acquisition gradient echo) dataset was acquired for anatomic definition. Ninety-three echo-planar functional datasets were obtained. The sagittal T1-weighted structural images were reformatted into approximately 61 sections with the same obliquity and anatomic coverage as the echo-planar images to display the functional data overlaid on the anatomic dataset.
Data and Statistical Analysis
Data were analyzed by using Statistical Parametric Mapping software (SPM99; Wellcome Department of Cognitive Neurology, London, UK) implemented in MatLab 6.0 (Mathworks Inc, Sherborn, MA) (30). Data were prepared for statistical analysis within SPM99. Of the 93 sets of brain images, the first three were discarded to avoid saturation effects. Hence, 90 sets were examined. To correct for motion, the 90 temporal datasets were spatially realigned within SPM99 by using a least-squares approach to estimate a six-parameter rigid-body transformation for each. The first dataset retained for analysis was used as a reference for realignment. The temporal datasets were normalized to a standard space (Montreal Neurologic Institute [MNI] space) within SPM99. This space was based on a template T1-weighted dataset, a close analog of Talairach space (31) developed by the MNI (supplied to SPM by Alan Evans, MNI, Canada [International Consortium for Brain Mapping, National Institutes of Health P20 project, principal investigator John Mazziotta]). The temporal datasets were smoothed by using an isotropic Gaussian filter kernel having a full-width at half maximum (FWHM) of twice the normalized voxel size.
Statistical analysis was accomplished within SPM99. Statistical parametric maps were generated by using the general linear model to characterize regionally specific effects in the imaging data. Terms in the model included the activity conditions (language task vs control) and the global mean value of each temporal dataset. A boxcar reference waveform convolved with a kernel that approximates the hemodynamic response curve was used to test specific hypotheses, resulting in a t value (SPM {t}) at each voxel. Within SPM, each SPM{t}statistic was transformed to the unit normal distribution to give an SPM{Z} statistic.
For all four tasks, statistical thresholding at a significance level of P < .001 (without correction for multiple comparisons) was initially used to determine significant activation for each voxel. To reduce the effect of type I error (spurious activation related to motion or other systematic error), a 10-voxel clustering (spatial-extent) threshold was applied so that only clusters consisting of 10 or more contiguous activated voxels were considered significant.
Cerebellar ROI analysis was performed on the SPM maps. Standardized coordinates were chosen for individual ROI analysis by visual inspection of the anatomic regions on orthogonal normalized SPM structural displays. A spherical ROI with a 25-mm radius was chosen. This was centered with center MNI coordinates of (−24, −63, −35) in the left cerebellar hemisphere and (24, −63, −35) in the right cerebellar hemisphere.
Voxel counts were tabulated for ROIs in each subject for fMRI results obtained at the P < .001 activation threshold. These voxel counts represented individual normalized activation data points for each anatomic ROI. Region-specific laterality indices were calculated as follows: Laterality index = (no. of activated voxels in left hemispheric ROI − no. of activated voxels in the homologous right hemispheric ROI)/(no. of activated voxels in the left hemispheric ROI + no. of activated voxels in the right hemispheric ROI). Two-way analysis of variance (ANOVA) with language and task as the two factors and with repeated measures on both factors was performed to study the relative contributions of the hemispheres to overall cerebellar activation.
In addition, group-level analysis was performed within SPM99 and based on voxels across all eight subjects with significant activation at P < .001. The purpose was to qualitatively assess differences in regional topography and the intensity of activation between similar language tasks performed in the two languages. In analyzing the results, pairs of tasks were considered. The Spanish noun-verb task was subtracted from the English noun-verb task, and the Spanish phonological task was subtracted from the English phonological task; the reverse subtractions were also performed. These results were displayed in three orthogonal planes as glass-brain maximum intensity projections (MIPs), which displayed the voxels across all eight subjects that were significantly more activated in the first of each pair of tasks than in the second; that is, they displayed the additional brain activation in the first task compared with second. Group-averaged normalized results for the combined tasks (English noun-verb and phonological, Spanish noun-verb and phonological) versus control were generated and displayed at a threshold of P < .001 with a 10-voxel clustering threshold. The combined Spanish tasks were then subtracted from the combined English tasks.
In addition, to evaluate the actual intensity of activation for individual subjects, we tabulated the absolute T values (T value maxima) of voxels with maximal activation in each cerebellar ROI (P < .001, 10-voxel clustering threshold). We also tabulated the voxel counts for single clusters of activated voxels (P < .001 activation threshold) containing the voxel with maximal T value in each ROI (modified voxel count). ANOVA procedures were then performed on the basis of these tabulated T valve maxima and modified voxel counts for each of the eight individuals in the study.
Two-way ANOVA with repeated measures on both factors (language and task) was performed on the response accuracy data obtained as a subject task-performance measure.
Results
The cerebellar fMRI activation data are summarized in the Table (Table 1).
Cerebellar ROI fMRI activation data
| Task | Voxel Count |
Left ROI |
Right ROI |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates of Voxel with Maximal T Value |
Maximal T Value | Modified Voxel Count | Coordinates of Voxel with Maximal T Value |
Maximal T Value | Modified Voxel Count | |||||||
| Left | Right | x | y | z | x | y | z | |||||
| Subject A | ||||||||||||
| ENG NnVb | 368 | 169 | −34 | −84 | −28 | 8.61 | 199 | 6 | −78 | −38 | 7.02 | 51 |
| ENG Phono | 403 | 100 | −40 | −62 | −26 | 8.11 | 256 | 8 | −76 | −28 | 5.31 | 22 |
| SPN NnVb | 155 | 68 | −32 | −84 | −26 | 6.75 | 85 | 18 | −80 | −40 | 4.85 | 30 |
| SPN Phono | 194 | 0 | −40 | −60 | −26 | 6.31 | 157 | 0 | 0 | |||
| Subject B | ||||||||||||
| ENG NnVb | 1223 | 1078 | −40 | −76 | −22 | 17.81 | 1084 | 36 | −74 | −20 | 11.9 | 669 |
| ENG Phono | 1175 | 1018 | −40 | −68 | −18 | 15.55 | 1067 | 38 | −74 | −26 | 10.31 | 683 |
| SPN NnVb | 387 | 186 | −38 | −76 | −20 | 12.59 | 272 | 28 | −82 | −28 | 5.33 | 21 |
| SPN Phono | 383 | 210 | −28 | −82 | −20 | 11.89 | 357 | 36 | −74 | −16 | 6.59 | 210 |
| Subject C | ||||||||||||
| ENG NnVb | 148 | 55 | −28 | −70 | −22 | 5.79 | 50 | 12 | −74 | −24 | 4.31 | 26 |
| ENG Phono | 185 | 59 | −48 | −58 | −30 | 6.85 | 74 | 18 | −54 | −12 | 4.96 | 6 |
| SPN NnVb | 240 | 87 | −18 | −58 | −12 | 5.66 | 26 | 40 | −52 | −22 | 4.53 | 36 |
| SPN Phono | 86 | 43 | −40 | −76 | −30 | 4.88 | 31 | 34 | −70 | −26 | 4.47 | 14 |
| Subject D | ||||||||||||
| ENG NnVb | 542 | 414 | −26 | −84 | −32 | 9.23 | 359 | 26 | −84 | −26 | 11.25 | 276 |
| ENG Phono | 309 | 426 | −24 | −86 | −34 | 7.23 | 262 | 28 | −84 | −26 | 11.91 | 223 |
| SPN NnVb | 227 | 32 | −42 | −74 | −26 | 6.28 | 139 | 44 | −60 | −20 | 4.12 | 5 |
| SPN Phono | 46 | 131 | −42 | −76 | −24 | 4.25 | 29 | 24 | −84 | −25 | 6.92 | 110 |
| Subject E | ||||||||||||
| ENG NnVb | 402 | 115 | −36 | −52 | −30 | 7.94 | 388 | 16 | −78 | −24 | 4.9 | 92 |
| ENG Phono | 346 | 163 | −36 | −78 | −22 | 9.06 | 172 | 22 | −76 | −26 | 4.85 | 117 |
| SPN NnVb | 167 | 13 | −34 | −76 | −24 | 5.99 | 99 | 26 | −80 | −32 | 4.53 | 13 |
| SPN Phono | 87 | 18 | −46 | −66 | −24 | 5.08 | 22 | 14 | −82 | −24 | 3.9 | 17 |
| Subject F | ||||||||||||
| ENG NnVb | 1 | 0 | −42 | −48 | −24 | 3.64 | 1 | 0 | 0 | |||
| ENG Phono | 285 | 248 | −36 | −64 | −30 | 6.55 | 124 | 22 | −74 | −20 | 6.69 | 175 |
| SPN NnVb | 120 | 118 | −38 | −48 | −32 | 5.45 | 77 | 40 | −74 | −22 | 6.46 | 71 |
| SPN Phono | 1 | 0 | −42 | −48 | −24 | 3.64 | 1 | 0 | 0 | |||
| Subject G | ||||||||||||
| ENG NnVb | 985 | 448 | −44 | −64 | −26 | 10.56 | 840 | 20 | −80 | −50 | 8.02 | 63 |
| ENG Phono | 401 | 391 | −46 | −58 | −26 | 7.36 | 327 | 38 | −76 | −36 | 7.27 | 110 |
| SPN NnVb | 71 | 12 | −46 | −58 | −26 | 5.33 | 38 | 6 | −76 | −32 | 4 | 12 |
| SPN Phono | 90 | 26 | −46 | −60 | −28 | 4.98 | 86 | 40 | −58 | −24 | 4.21 | 23 |
| Subject H | ||||||||||||
| ENG NnVb | 233 | 118 | −38 | −80 | −24 | 5.71 | 206 | 16 | −74 | −40 | 4.74 | 76 |
| ENG Phono | 338 | 112 | −42 | −74 | −24 | 6.72 | 310 | 20 | −72 | −36 | 5.84 | 83 |
| SPN NnVb | 279 | 139 | −46 | −66 | −26 | 5.68 | 150 | 28 | −74 | −30 | 6.07 | 73 |
| SPN Phono | 17 | 0 | −42 | −66 | −22 | 4.61 | 17 | 0 | 0 | |||
Note.—ENG = English, SPN = Spanish, NnVb = noun-verb association task, Phono = phonological task.
Multiple two-way ANOVA procedures with repeated measures on the two factors (language and task) were performed for computed total ROI voxel count laterality index (LI) data based on individual subject voxel counts obtained at an activation threshold of P < .001 (with a 10-voxel clustering threshold applied) for the cerebellar ROIs. The mean Spanish laterality index of 0.3286 was significantly different from the mean English laterality index of 0.5141 in the cerebellar ROIs (P = .0143). However, no task-specific differences were noted (P = .8068), and no significant language * task interaction effect (P = .4184) was demonstrated.
ANOVA of T value maxima demonstrated a significant difference between the English (7.6878 ± 3.4708 [mean ± SD]) and Spanish (5.1672 ± 2.5674) language tasks (P = .0018). However, the maxima did not differ between semantic and phonological tasks (P = .1107). These findings suggested more robust overall activation in the English tasks than in the Spanish tasks. ANOVA revealed a similar finding with the modified voxel counts. Counts in the English tasks were significantly different from those in the Spanish tasks (P = .0055), but no task effect was present (P = .5229). This finding, which described the spatial extent of activation, indicated more robust overall cerebellar activation in the English tasks than in the Spanish tasks.
Two-way ANOVA with repeated measures revealed a significant language effect (P = .0001) but no task effect (P = .067). The subjects had a significantly greater error rate in the English tasks (9.25 ± 4.60 incorrect responses from 93 total) than in the Spanish tasks (1.00 ± 0.894 incorrect responses). However, the overall mean percentage of correct responses for both English and Spanish tasks was over 90% (average, 83.75 correct responses per individual in English vs 92 in Spanish). This finding may have been related to differences in task difficulty or English proficiency.
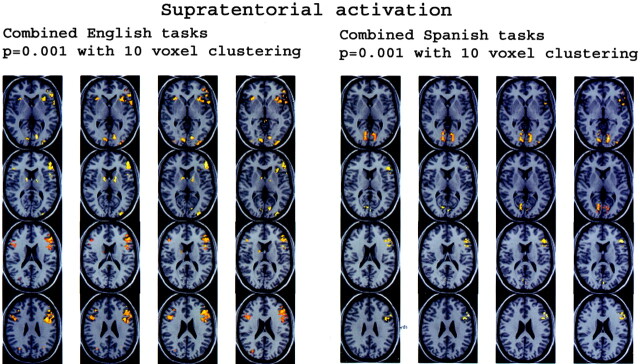
Figure 1 displays four sets of orthogonal glass-brain MIP images for the group-averaged task subtractions. Figure 2 shows the combined language task subtractions. Figure 3 displays axial normalized anatomic overlay images of the English and Spanish noun-verb tasks. This Figure demonstrates graphically the difference in lateralization between the English and Spanish noun-verb tasks, with demonstration of greater degree of left cerebellar hemispheric lateralization in the English noun-verb task. Figure 4 illustrates the combined English and Spanish tasks (vs control). These results are derived from a single sample t-test, which is based on consideration of each of the two tasks (English noun-verb and English phonological for Part A and Spanish noun-verb and Spanish phonological for Part B). Figure 5 depicts anatomic overlays of the functional data from the combined English and combined Spanish tasks in Figure 4 on T1-weighted MPRAGE images. In this Figure, only anatomic sections at the level of the cerebellum are displayed. Lastly, Figure 6 displays additional anatomic overlays of the group-averaged normalized combined English task and combined Spanish task functional data in supratentorial anatomic sections.
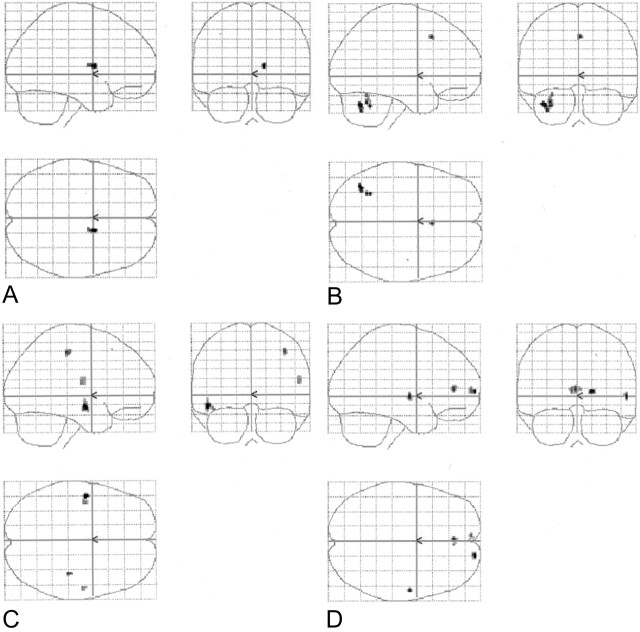
Fig 1.
Four sets of orthogonal SPM99 glass-brain MIP images depict the normalized group-averaged results of all eight individuals (P = .001, 10-voxel clustering threshold). On the coronal images, the left side represents the left hemisphere. On the axial images, the left side is posterior, and the lower half represents the right hemisphere.
A, Spanish noun-verb task subtracted from the English noun-verb task.
B, Spanish phonological task subtracted from the English phonological task.
C, English noun-verb task subtracted from the Spanish noun-verb task.
D, English phonological task subtracted from the Spanish phonological task.
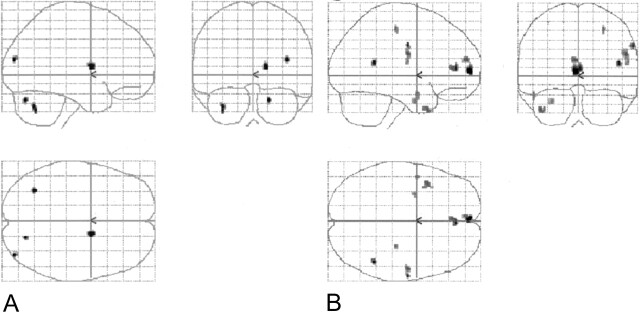
Fig 2.
Combined (phonological and noun-verb) task subtractions. Orthogonal glass-brain MIP images display group-averaged normalized results (P < .001, 10-voxel clustering threshold).
A, English minus Spanish.
B, Spanish minus English.
Fig 3.
Group-averaged normalized data for all eight subjects. Activated voxels are overlaid on standard T1-weighted axial anatomic images, which demonstrate the difference in lateralization between the tasks, with greater left lateralization in the English task. The datasets were normalized to the standard MNI space within SPM99. Top row, Spanish noun-verb task. Bottom row, English noun-verb task.
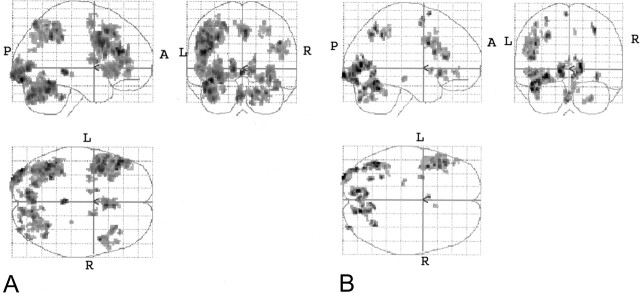
Fig 4.
Combined tasks. These results are essentially based on a single sample t test based on a consideration of individual normalized data for each of the two tasks (P < .001 with 10-voxel clustering).
A, Combined English tasks versus control.
B, Combined Spanish tasks versus control.
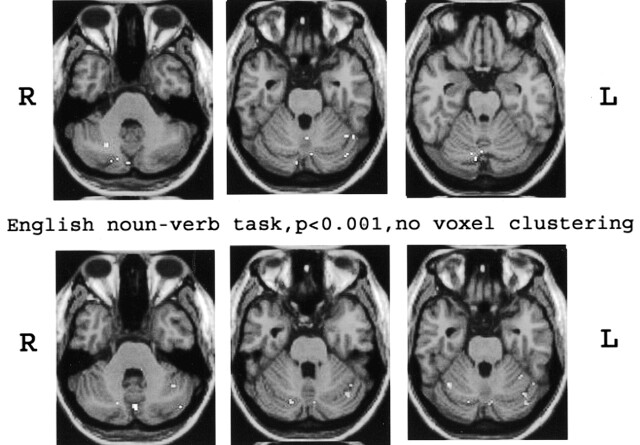
Fig 5.
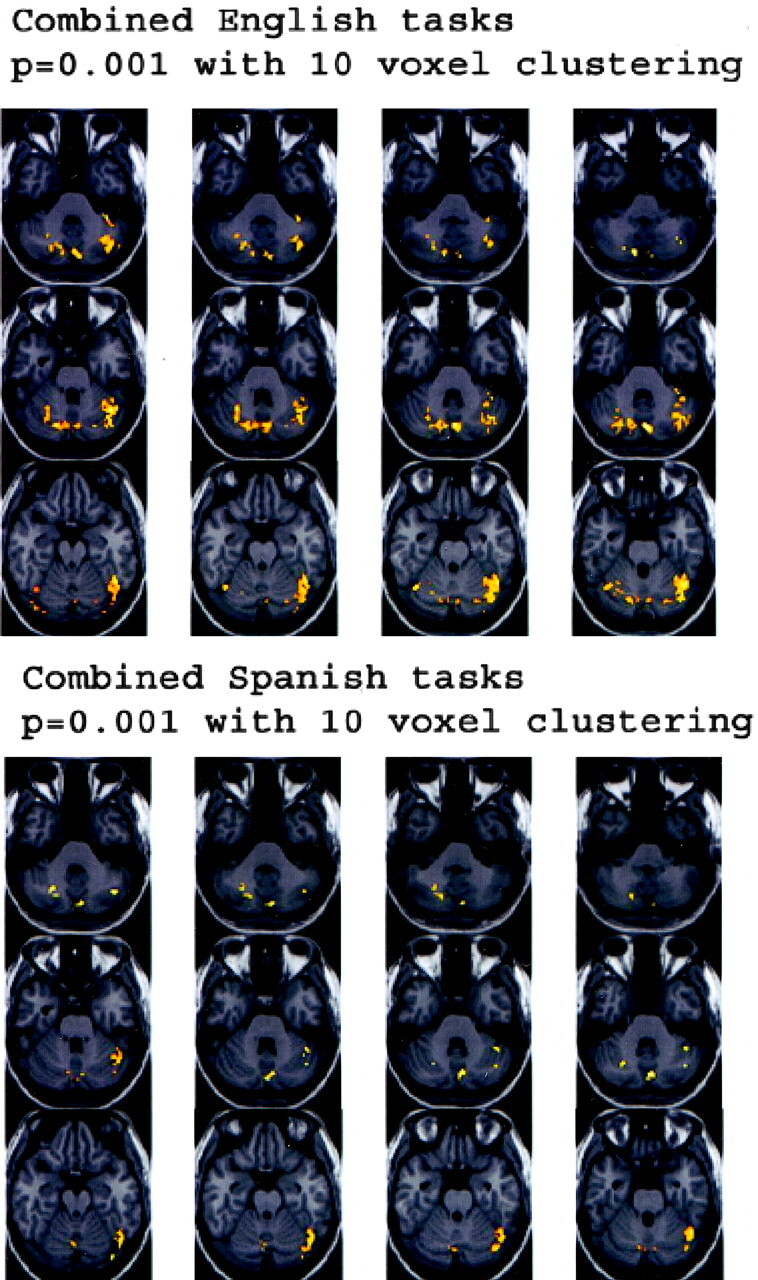
Anatomic MPRAGE images show the functional data overlays on the anatomic sections through the cerebellum. The results from Figure 4A (top) and B (bottom) are depicted. The right side of each image is the anatomic left side, and the left side of each image, the anatomic right side.
Fig 6.
Anatomic overlays of the group-averaged normalized combined English task and combined Spanish task functional data on supratentorial anatomic MPRAGE sections. Functional data from Figure 5 are shown (P < .001 with 10 voxel clustering).
Group results for the individual language task versus control were reported in a previous publication (28) and not discussed here.
Discussion
Although the role of the cerebellum was considered to be limited to only motor function involving the muscles of articulation, it is now clear that the cerebellum plays a important role in cognition, particularly language processing. The cerebellum is believed to have a mostly modulatory role because its extensive anatomic connections with the thalamus and cerebral cortices (especially the frontal and parietal lobes) (23–26).
Lesional studies in monolingual individuals with cerebellar structural abnormalities (eg, stroke, tumors, infection) have demonstrated that the right cerebellar hemisphere, the left cerebellar hemisphere, and the cerebellar vermis serve different functions. Activation studies have confirmed the results of the original PET study by Petersen et al (32–36), which demonstrated activation in the inferior lateral aspect of the right cerebellar hemisphere (which projects to the left prefrontal language area) in a verbal association task. However, activation was seen in the superior anterior lobe of the cerebellum just lateral to the loci involved in finger and eye movements during a simple verbal-motor task. Marien and colleagues (37) performed an excellent review of the literature, tabulating various language functions attributable to the right and left cerebellar hemispheres. Lesions in the left cerebellar hemisphere have been associated with loss of muscular speech control resulting in ataxic dysarthria and with loss of visuospatial organization resulting in afferent dysgraphia (38–41). Numerous studies demonstrate a greater overall role of the right cerebellar hemisphere in language functions (37). The functions of covert articulation, articulatory planning (dysfunction resulting in speech apraxia), verbal associations, word rhyming, generation of synonyms, word completion, semantic associations, phonological generation, expressive grammar, syntactic knowledge, and language dynamics have all been associated with the right cerebellar hemisphere.
Schmahmann et al (42–44)and others (37, 45) describe a complex functional topography of the cerebellum, providing evidence for at least three functionally distinct cerebellar areas: 1) the sensorimotor cerebellum rostral to the primary fissure in the anterior lobe with a secondary representation in lobules VII/IX of the cerebellar hemispheres; 2) the cognitive cerebellum (also referred to as the neocerebellum) consisting of the lateral cerebellar hemispheres and the dentate and emboliform nuclei) in lobule VI and VII at the vermis with extension into lobule VI, crus I and II of lobule VIIA and lobule VIIB of the cerebellar hemispheres; and 3) the limbic cerebellum that encompasses the more primitive cerebellar fastigial nucleus, the vermis, and the flocculonodular lobes. Riva and Giorgi (45) confirmed many of these topographic differences in their study of 14 children with cerebellar hemispheric lesions, 11 children with vermian lesions, and one child with viral cerebellitis. Lesions of the vermis (particularly the lower lobules) produced behavioral disturbances ranging from irritability to psychosis. Right hemispheric lesions impaired language processing, symbolic sequencing, problem solving, and categorical memory, whereas left hemispheric lesions produced losses in speech melody, fluency of designs, and visual sequential memory. Furthermore, their results demonstrated that the connections between the cerebellum and the frontal associative cortical areas become functional early in life, certainly by the age of 3 years. The increased plasticity of the brain in childhood was believed to be insufficient to compensate for early cerebellar lesions.
The phenomenon of crossed cerebellocerebral diaschisis, first described by Broich and colleagues in 1987 (46), now refers to a well-recognized pattern of perfusion deficits in patients with strokes. Broich et al reported a patient with a right cerebellar hemispheric infarct that produced remote, contralateral left hemispheric hypoperfusion with greatest hypometabolism in the premotor areas. Since then, numerous reports of this phenomenon have arisen, and crossed cerebellocerebral diaschisis has been described in patients with unilateral cerebellar stroke, neurodegenerative disorders, and neurocognitive and neurolinguistic deficits (23, 47–59). This phenomenon supports the hypothesis that the left frontal lobe and the right cerebellar hemisphere are functionally and closely connected.
Bilingual studies of supratentorial activation have demonstrated increased right hemispheric (frontal) activation in the non-native language compared with the native language. Although most work in the fMRI evaluation of language processing (semantic, phonological, and other) has demonstrated predominantly left hemispheric areas of supratentorial activation (inferior frontal gyrus, dorsolateral prefrontal cortex, superior temporal gyrus, supramarginal gyrus, etc), several studies show that poorly to moderately proficient subjects who acquire L2 late have a greater tendency to display right cerebral hemispheric activation in various language tasks. Calabrese et al (1) have shown that, while predominantly left prefrontal activation is present during both L1 and L2 processing in a word fluency paradigm, right prefrontal activation is also present during L2 processing. In another study, moderately fluent French (L1) and English (L2) bilingual subjects listened to stories in both languages (6). Left hemispheric activation (left superior temporal sulcus) was consistently seen during the L1 version of the language comprehension task, but variable bihemispheric activation (involving bilateral temporal and frontal regions) was seen during the L2 comprehension task. In their study of English-Mandarin bilingual persons, Chee and colleagues (5) noted bilateral inferior frontal activation in some and unilateral (left) prefrontal and parietal activation in others. They attributed the right hemispheric activation to low L2 proficiency because more-proficient individuals displayed more left lateralized activation. In our previous article (28), we noted greater right hemispheric activation (right frontal lobe activation) in the English phonological task than with the Spanish phonological task. We attributed this finding (at least partly) to our subjects’ moderate L2 proficiency or to the relatively late age at which they acquired L2. In addition, the higher cognitive demands of task performance in their non-native language may also account for the decreased lateralized supratentorial activation in the English tasks as compared with the Spanish tasks. However, none of the previously reported fMRI (or PET) studies of bilingual subjects specifically examined cerebellar language activation or compared cerebellar activation patterns in L1 and L2.
Our results demonstrate greater contribution of the left cerebellar hemisphere to overall cerebellar activation in the non-native language (English) than with the native language (Spanish). This finding may have been secondary to crossed cerebellar diaschisis. As described in our previous work on supratentorial activation in this group (28), greater right hemispheric supratentorial activation is observed in L2 as compared with L1. The frontocerebellar pathways may be responsible. However, the few reports about cerebellar activation in the bilingual literature seem to indicate only right cerebellar activation in language tasks (performed in L1 or L2) in bilingual persons (14, 60). This may be related to the method of stimulus presentation. In our study, visual stimuli were used throughout, whereas in other studies, auditory or other stimuli were used. It is clear from previous work in patients with cerebellar lesions that the left cerebellar hemisphere is involved in visuospatial processing. In addition, although common language networks may be used for both processing in L1 and L2, perhaps greater utilization of certain subsystems or use of entirely different subsystems is responsible for the differences in activation topography on the task-subtraction activation maps. Variations in our task design from those of other fMRI studies in bilingual subjects may account for some of the differences observed.
The presence of left-lateralized cerebellar activation in right-handed individuals during the performance of both tasks in L1 and L2 is surprising and not in accordance with results from the few previously published studies. Crossed cerebellar diaschisis does not account for this finding because no significant supratentorial right hemispheric activation was seen in L1 in our previous work.
The possibility of motor contamination must be addressed in any study of cerebellar activation, particularly when finger presses on a keypad are used to record responses to stimuli. In our study, this possibility can easily be discounted because, in both the activation (language) and control (nonsense drawing) tasks, equal numbers of correct right- and left-hand responses were devised to effectively cancel the motor component from the activation. Incorrect responses are expected to occur randomly, and in the process of creating group-averaged results, any skewing of results in favor of greater motor activation for one hand or the other should be effectively minimized.
Limitations of the study include the relatively small sample, which reduces its overall statistical power. In addition, the presence of a sex-skewed sample limits any evaluation of sex-related effects on language activation. Furthermore, the presence of important linguistic differences between Spanish and English, such as word-order specificity and word morphology, might have affected fMRI task activation. More research is necessary to study the effects of differences in linguistic structure on language representation and processing. The lack of reproducibility data for these specific tasks in this particular cohort is another limitation of this preliminary study. Although we have examined interimaging variability with English versions of these paradigms, we have not yet done so with the Spanish tasks. Lastly, despite the presence of response accuracy data, homogeneous educational demographics, and use of subjective ratings of language proficiency, the lack of objective language proficiency measures (eg, those of the Boston Naming Test) is a limitation. For future investigations, such objective measurements should be used to avoid a potential confounding subject-proficiency variable.
Despite these limitations, our results indicate that the cerebellar hemispheres participate in language processing and also that the extent and laterality of activation differ between native and non-native languages. Whether this reflects differential utilization of a common, bilateral language neural network in the cerebellar hemispheres or different contributions of the cerebellar hemispheres in modulating the supratentorial language network is unclear. Investigations of the exact nature of cerebellar activation in language processing need to be performed. Research in bilingual individuals is particularly useful to elucidate the role of the cerebellum in cognition and to gain insights into the acquisition and maintenance of linguistic functions. As the phenomenon of cerebellocerebral diaschisis demonstrates, the cerebellum and supratentorial language-processing centers share intimate connections. Knowledge of how these connections operate and how the cerebellum participates in overall language processing is vital to understanding the effects of neurologic disease on language function; this, in turn, is crucial for designing adequate speech rehabilitation for patients with stroke or cerebellar neoplasia.
Conclusions
Our preliminary data demonstrate significantly greater left lateralization of cerebellar language activation with the non-native language compared with the native language. However, overall left cerebellar hemispheric lateralization was seen during task performance in both languages. Future studies of bilingual individuals are needed to investigate the exact role the normal cerebellum in the modulation of complex cerebellocerebral neural circuitry and to better predict functional recovery of language in patients with cerebellar disease.
Footnotes
Supported in part by a 2000 Phillips Medical Systems/Radiological Society of North America Seed grant.
Presented at the 41st Annual Meeting of the American Society of Neuroradiology; Washington, DC, April 29, 2003.
References
- 1.Calabrese P, Neufeld H, Falk A, et al. Word generation in bilinguals—fMRI study with implications for language and memory processes [German]. Fortschritte der Neurologie-Psychiatrie 2001;69(1):42–49 [DOI] [PubMed] [Google Scholar]
- 2.Chee MW, Caplan D, Soon CS, et al. Processing of visually presented sentences in Mandarin and English studied with fMRI. Neuron 1999a;23(1):127–137 [DOI] [PubMed] [Google Scholar]
- 3.Chee MWL, Tan EWL, Thiel T. Mandarin and English single word processing studied with functional magnetic resonance imaging. J Neurosci 1999b;19:3050–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chee MW, Weekes B, Lee KM, et al. Overlap and dissociation of semantic processing of Chinese characters, English words, and pictures: evidence from fMRI. Neuroimage 2000;12(4):392–403 [DOI] [PubMed] [Google Scholar]
- 5.Chee MW, Hon N, Lee HL, Soon CS. Relative language proficiency modulates BOLD signal change when bilinguals perform semantic judgments. Neuroimage 2001;13(6 pt 1):1155–1163 [DOI] [PubMed] [Google Scholar]
- 6.Dehaene S, Dupoux E, Mehler J, et al. Anatomical variability in the cortical representation of first and second language. NeuroReport 1997;8:3809–3815 [DOI] [PubMed] [Google Scholar]
- 7.Hernandez AE, Martinez A, Kohnert K. In search of the language switch: An fMRI study of picture naming in Spanish-English bilinguals. Brain Lang 2000;73(3):421–431 [DOI] [PubMed] [Google Scholar]
- 8.Hernandez AE, Dapretto M, Mazziotta J, Bookheimer S. Language switching and language representation in Spanish-English bilinguals: an fMRI study. Neuroimage 2001;14(2):510–520 [DOI] [PubMed] [Google Scholar]
- 9.Illes J, Francis WS, Desmond JE, et al. Convergent cortical representation of semantic processing in bilinguals. Brain Lang 1999;70(3):347–363 [DOI] [PubMed] [Google Scholar]
- 10.Kim KHS, Relkin NR, Lee KM, Hirsch J. Distinct cortical areas associated with native and second languages. Nature 1997;388(6638):171–174 [DOI] [PubMed] [Google Scholar]
- 11.Klein D, Zatorre RJ, Milner B, Meyer E. Left putaminal activation when speaking a second language: evidence from PET. NeuroReport 1994;5(17):2295–2297 [DOI] [PubMed] [Google Scholar]
- 12.Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC. The neural substrates underlying word generation: a bilingual functional-imaging study. Proc Natl Acad Sci U S A 1995;92:2899–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakada T, Fujii Y, Kwee IL. Brain strategies for reading in the second language are determined by the first language. Neurosci Res Suppl 2000;40(4):351–358 [DOI] [PubMed] [Google Scholar]
- 14.Perani D, Dehaene S, Grassi F, et al. Brain processing of native and foreign languages. NeuroReport 1996;7:2439–2444 [DOI] [PubMed] [Google Scholar]
- 15.Perani D, Paulesu E, Galles NS, et al. The bilingual brain: proficiency and age of acquisition of the second language. Brain 1998;121(pt 10):1841–1852 [DOI] [PubMed] [Google Scholar]
- 16.Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage 1999;10:15–35 [DOI] [PubMed] [Google Scholar]
- 17.Simos PG, Castillo EM, Fletcher JM, et al. Mapping of receptive language cortex in bilingual volunteers by using magnetic source imaging. J Neurosurg 2001;95:76–81 [DOI] [PubMed] [Google Scholar]
- 18.Albert ML, Obler LK. The Bilingual Brain: Neuropsychological and Neurolinguistic Aspects of Bilingualism. New York: Academic Press;1978
- 19.Gomez-Tortosa E, Martin EM, Gaviria M, Charbel F, Ausman JI. Selective deficit of one language in a bilingual patient following surgery in the left perisylvian area. Brain Lang 1995;48(3):320–325 [DOI] [PubMed] [Google Scholar]
- 20.Kircher TT, Brammer M, Tous Andreu N, Williams SC, McGuire PK. Engagement of right temporal cortex during processing of linguistic context. Neuropsychologia 2001;39(8):798–809 [DOI] [PubMed] [Google Scholar]
- 21.Ojemann GA. Brain organization for language from the perspective of electrical stimulation mapping. Behav Brain Sci 1983;6:189–206 [Google Scholar]
- 22.Paradis M. Aspects of Bilingual Aphasia. Oxford: Pergamon;1995
- 23.Marien P, Engelborghs S, DeDeyn PP. Cerebellar neurocognition: a new avenue. Acta Neurol Belg 2001;101:96–109 [PubMed] [Google Scholar]
- 24.Engelborghs S, Marien P, Martin JJ, DeDeyn PP. Functional anatomy, vascularisation and pathology of the human thalamus. Acta Neurol Belg 1998;98:252–265 [PubMed] [Google Scholar]
- 25.Leiner HC, Leiner AL, Dow RS. Does the cerebellum contribute to mental skills? Behav Neurosci 1986;100:443–454 [DOI] [PubMed] [Google Scholar]
- 26.Leiner HC, Leiner AL, Dow RS. Reappraising the cerebellum: what does the hindbrain contribute to the forebrain? Behav Neurosci 1989;103:998–1008 [DOI] [PubMed] [Google Scholar]
- 27.Varney NR, Benton AL. Tactile perception of direction in relation to handedness and familial handedness. Neuropsychologia 1975;13(4):449–454 [DOI] [PubMed] [Google Scholar]
- 28.Pillai JJ, Araque JM, Allison JD, et al. Functional MRI study of semantic and phonological language processing in bilingual subjects: preliminary findings. NeuroImage 2003;19(3):565–576 [DOI] [PubMed] [Google Scholar]
- 29.Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: a new interactive environment for designing psychology experiments. Behav Res Methods Instru Comput 1993;25(2):257–271 [Google Scholar]
- 30.Friston KJ. Testing for anatomically specified regional effects. Hum Brain Mapping 1997. :5(2):133–136 [DOI] [PubMed] [Google Scholar]
- 31.Tailarach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical;1988
- 32.Petersen SE, Fox PT, Posner MI, Mintun MA, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature 1988;331:585–589 [DOI] [PubMed] [Google Scholar]
- 33.Petersen SE, Fox PT, Posner MI, Mintun MA, Raichle ME. Positron emission tomographic studies of the processing of single words. J Cogn Neurosci 1989;1:153–170 [DOI] [PubMed] [Google Scholar]
- 34.Raichle ME, Fiez JA, Videen TO, et al. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex 1994;4:8–26 [DOI] [PubMed] [Google Scholar]
- 35.Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science 1995;270:102–105 [DOI] [PubMed] [Google Scholar]
- 36.Grabowski TJ, Frank RJ, Brown CK, et al. Reliability of PET activation across statistical methods, subject groups, and sample sizes. Hum Brain Mapping 1996;4:23–46 [DOI] [PubMed] [Google Scholar]
- 37.Marien P, Engelborghs S, Fabbro F, De Deyn PP. The lateralized linguistic cerebellum: a review and a new hypothesis. Brain Lang 2001;79:580–600 [DOI] [PubMed] [Google Scholar]
- 38.Holmes G. The symptoms of acute cerebellar injuries due to gunshot injuries. Brain 1917;40:401–534 [Google Scholar]
- 39.Lectenberg R, Gilman S. Speech disorders in cerebellar disease. Ann Neurol 1978;3:285–290 [DOI] [PubMed] [Google Scholar]
- 40.Silveri MC, Misciagna S, Leggio MG, Molinari M. Spatial dysgraphia and cerebellar lesion: a case report. Neurology 1997;48:1529–1532 [DOI] [PubMed] [Google Scholar]
- 41.Silveri MC, Misciagna S, Leggio MG, Molinari M. Cerebellar spatial dysgraphia: further evidence. J Neurol 1999;246:321–323 [DOI] [PubMed] [Google Scholar]
- 42.Schmahmann JD, Loeber RT, Marjani J, Hurwitz AS. Topographic organization of cognitive function in the human cerebellum: a meta-analysis of functional imaging studies. NeuroImage 1998;7:S721 [Google Scholar]
- 43.Schmahmann JD, Doyon J, McDonald D, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage 1999;10:233–260 [DOI] [PubMed] [Google Scholar]
- 44.Schmahmann JD. The role of the cerebellum in affect and psychosis. J Neuroling 2000;13:189–214 [Google Scholar]
- 45.Riva D, Giorgi C. The contribution of the cerebellum to mental and social functions in developmental age. Fiziol Cheloveka. 2000;26(1):27–31 [PubMed] [Google Scholar]
- 46.Broich K, Hartmann A, Biersck HJ, Horn R. Crossed cerebello-cerebral diaschisis in a patient with cerebellar infarction. Neurosci Lett 1987;83:7–12 [DOI] [PubMed] [Google Scholar]
- 47.Kimura S, Nakamura H, Matsumura K, et al. Crossed ‘cerebral’ diaschisis? Seven cases with unilateral cerebellar vascular lesions which showed decreased perfusion in the contralateral cortex. Kaku-Igaku 1989;26:1259–1266 [PubMed] [Google Scholar]
- 48.Yokoji H, Ide Y, Matsubara S, Takamori M. A case of cerebellar hemispheric venous malformation presenting crossed cerebello-cerebral diaschisis. Rinsho-Shinkeigaku 1989;29:1414–1416 [PubMed] [Google Scholar]
- 49.Rousseaux M, Steinling M. Crossed hemispheric diaschisis in unilateral cerebellar lesions. Stroke 1992;23:511–514 [DOI] [PubMed] [Google Scholar]
- 50.Botez-Marquard T, Botez MI. Cognitive behavior in heredodegenerative ataxias. Eur Neurol 1993;33:351–357 [DOI] [PubMed] [Google Scholar]
- 51.Sonmezoglu K, Sperling B, Henriksen T, Tfelt-Hansen P, Lassen NA. Reduced contralateral hemispheric flow measured by SPECT in cerebellar lesions: crossed cerebral diaschisis. Acta Neurol Scand 1993;87:275–280 [DOI] [PubMed] [Google Scholar]
- 52.Deguchi K, Takeuchi H, Yamada A, Touge T, Nishioka M. Rinsho-Shinkeigaku 1994;34:851–853 [PubMed] [Google Scholar]
- 53.Attig E, Botez MI, Hublet CI, Verdonck C, Jacquy J, Capon A. Cerebral diaschisis following cerebellar lesion: contribution of the cerebellum to cognitive functions. Rev Neurol 1991;147:200–201 [PubMed] [Google Scholar]
- 54.Boni S, Valle G, Gioffi RP, et al. Crossed cerebello-cerebral diaschisis: a SPECT study. Nucl Med Commun 1992;13:824–831 [DOI] [PubMed] [Google Scholar]
- 55.Botez-Marquard T, Leveille J, Botez MI. Neuropsychological functioning in unilateral cerebellar damage. Can J Neurol Sci 1994;21:353–357 [DOI] [PubMed] [Google Scholar]
- 56.Silveri MC, Leggio MG, Molinari M. The cerebellum contributes to linguistic production: a case of agrammatic speech following a right cerebellar lesion. Neurology 1994;44:2047–2050 [DOI] [PubMed] [Google Scholar]
- 57.Marien P, Saerens J, Nanhoe R, et al. Cerebellar induced aphasia: case report of cerebellar induced prefrontal aphasic language phenomena supported by SPECT findings. J Neurol Sci 1996;144:34–43 [DOI] [PubMed] [Google Scholar]
- 58.Marien P, Engelborghs S, Pickut BA, De Deyn PP. Aphasia following cerebellar damage: fact or fallacy? J Neuroling 2000;13:145–171 [Google Scholar]
- 59.Zettin M, Cappa SF, D’Amico A, et al. Agrammatic speech production after a right cerebellar haemorrhage. Neurocase 1997;3:375–380 [Google Scholar]
- 60.Klein D, Milner B, Zatorre RJ, Zhao V, and Nikelski J. Cerebral organization in bilinguals: a PET study of Chinese-English verb generation. NeuroReport 1999;10:2841–2846 [DOI] [PubMed] [Google Scholar]