Abstract
BACKGROUND AND PURPOSE: Patients with traumatic brain injury (TBI) and low Glasgow coma scale (GCS) scores may have severe injury associated with cellular disruption that can be studied with whole-brain apparent diffusion coefficient (ADC) histograms. We retrospectively studied this hypothesis and correlated ADC with GCS.
METHODS: Twenty-one patients (37.81 ± 41.3 years) with TBI were enrolled: Group A had normal MR imaging findings but low GCS scores (n = 6). Group B had brain stem injury with low GCS scores (n = 6). Group C had cortical lesions with normal GCS scores (n = 5), and group D had cortical lesions with low GCS scores (n = 4). Eleven control subjects were enrolled (32.7 ± 19.2 years). Whole-brain ADC maps and histograms were generated and normalized for each subject. Mean and peak ADCs were determined. A one-sided t test was performed for each parameter. Average GCS scores and corresponding peak and mean ADCs were correlated.
RESULTS: Peak histogram values significantly differed between controls and groups A, B, and D (P < .0019, P < .00129, and P < .0148, respectively). In groups A and D, values were significantly skewed compared with control values. Mean ADC was significantly different between the control ADC and group A (P < .013) but not group C. In each group, peak ADC and GCS score were strongly correlated (R2 = 0.67).
CONCLUSION: Whole-brain peak ADCs and GCS scores are significantly correlated in patients with TBI. Although conventional MR images were normal, ADC independently indicated TBI and better represents the degree of neurologic dysfunction.
CT is the imaging technique of choice for initial evaluation of the extent of traumatic brain injury (TBI) in patients with blunt or penetrating trauma (1, 2). MR imaging is usually performed in the subacute period when CT results do not correlate with the neurologic findings. MR imaging is more sensitive than CT in demonstrating diffuse axonal shearing injuries, brain stem contusions or hemorrhage, and small extra-axial fluid collections (2). MR imaging findings can explain a particular neurologic deficit by demonstrating injuries that are occult on CT scans. They can also offer more accurate prognostic information.
The ability to study the change in the random motion of proton in water in vivo is the basis for diffusion-weighted (DW) MR imaging (1, 4–7). DW-MR imaging and tissue perfusion imaging has now become integral parts of the routine imaging studies performed to evaluate acute stroke (4–7). DW-MR imaging is more sensitive than conventional MR imaging in depicting restricted proton motion resulting from cytotoxic edema in ischemic brain tissue (1, 3, 4). Restricted proton motion results in a decrease in the apparent diffusion coefficient (ADC) of water and increased signal intensity on DW images.
DW-MR imaging has been studied in a variety of experimental animal models of TBI, with varying conclusions concerning expected changes in ADC values (8–13). Previous studies in patients with acute TBI have attempted to demonstrate changes in ADC values and tissue injury patterns (as visualized on DW-MR images) and also the ability to depict additional sites of injury not seen on conventional MR imaging (1, 14–16). However none of the studies to date have correlated the Glasgow Coma Scale (GCS) score with changes in ADC values or imaging findings. We investigated the hypothesis that patients with TBI and a low GCS score have severe brain injury associated with cellular disruption that can be studied by using whole-brain ADC histograms. We also attempted to determine if whole-brain ADC values indicate the presence of TBI in patients with abnormal GCS scores but normal conventional MR imaging results.
Methods
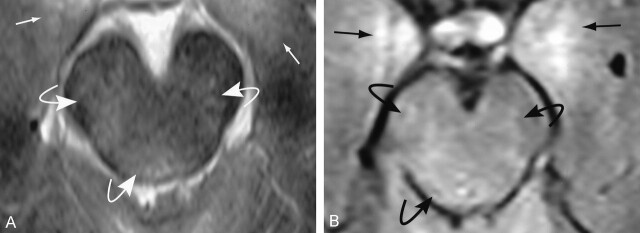
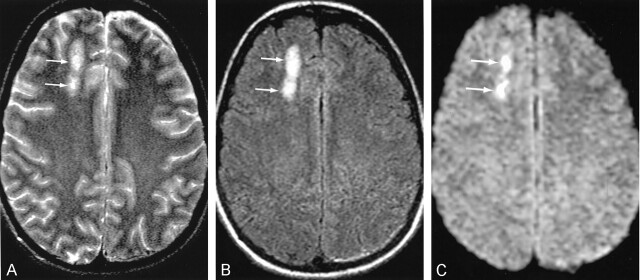
We evaluated data from 21 patients (age 37.81 ± 41.3 years; 14 men, seven women) who were admitted to our trauma center after blunt-force trauma with head injuries. Both conventional MR imaging and DW-MR imaging were performed as part of the standard protocol in all 21 patients. MR imaging was performed because the patients’ neurologic deficits or GCS score did not correlate with the CT findings (20 patients); in one patient MR imaging was performed to evaluate isolated subarachnoid hemorrhage following trauma. Mechanisms of injury included motor vehicle collisions (n = 10), assaults (n = 7), falls (n = 3), and a diving accident (n = 1). Patients with an abnormal GCS score as a result of alcohol or drug use, medical therapy, or illnesses were excluded. The study patients were divided into groups, as follows: Group A (n = 6; age 46 ± 24.6 years) had normal conventional MR imaging findings but low GCS scores (range, 6–10; mean, 8). Group B (n = 6; age 25 ± 12.15 years) had brain stem abnormalities (eg, hemorrhage in five patients), as shown on conventional MR images, with a low GCS score (range, 3–10; mean, 6) (Fig 1). Group C (n = 5; age, 36 ± 18.9 years) included patients whose lesions (eg, hemorrhage in four) were confined to the cortex on conventional MR images and whose GCS score was normal or near-normal (Fig 2). Group D (n = 4; age, 47 ± 34.4 years) patients had lesions similar to those in group C (eg, hemorrhage in two) but also had low GCS scores. A low GCS score was defined as an admission score of 10 or lower, and a near-normal or normal score was 13 or higher. Our institutional review board approved the study. Findings in the patients were compared with those of healthy control subjects (n = 11; age 32.7 ± 19.2 years; eight men, three women).
Fig 1.
Brain stem contusion in a 26-year-old man admitted after blunt-force trauma. Axial images show bilateral brain stem (curved arrows) and medial temporal (straight arrows) contusions in the brain parenchyma.
A, T2-weighted image.
B, FLAIR image.
Fig 2.
Minimal parenchymal contusions in a 36- year-old patient admitted after a head injury. Axial images show intraparenchymal contusions (arrows) in the right frontal lobe.
A, T2-weighted image.
B, FLAIR image.
C, DW image.
MR imaging was performed on one of two 1.5-T scanners: a Philips Eclipse unit (formerly Marconi Medical Systems, Cleveland, OH) or a Signa unit (GE Medical Systems, Milwaukee, WI). Mean interval to MR imaging after injury was 6 days (range, 1–23 days). The MR imaging protocol consisted of the following sequences covering the entire brain: a sagittal T1-weighted sequence (TR/TE, 450/11), an axial T1-weighted sequence (TR/TE, 450/11), an axial T2-weighted sequence (TR/TE, 5150/95), a fluid-attenuated inversion-recovery (FLAIR) sequence (TR/TI/TE 10000/2000/125), a delayed gradient-echo sequence (TR/TE, 595/20; flip angle, 30°), and DW imaging on all three orthogonal axis with a b value of 1000 sec/mm2. The FOV was kept constant in all the imaging sequences at 24 cm, and the section thickness was 5 mm with a 1-mm gap. Except for the DW images, other images were obtained at a resolution of 256 × 192. DW images were obtained at a reconstruction resolution of 128 × 128 over the same FOV as that of conventional sequences. Twenty-four sections covering the entire brain were obtained on the Eclipse machine, and 23 sections were obtained on the Signa unit. To assess the difference between the two machines, a standard head phantom treated with manganese chloride was used to measure the ADC of water in both the scanners by using the same sequence used to obtain ADC maps in the participants. Both machines yielded an ADC of about 2.07 × 10−5 cm2/s at a temperature of 25°C; the difference between them was less than 0.05%. Three radiologists (K.S., S.E.M., P.A.M.) reviewed the MR imaging studies and determined the findings by consensus.
The DW data consisting of DW images from all the three orientations along with a non-DW image set. These were transferred to an Ultra 80 workstation (Sun Microsystems, Palo Alto, CA). ADC maps were generated by using software developed in-house with MEDX image processing software (Sensor Systems, Sterling, VA). Computation of the ADC involved calculating the diffusion coefficient along each orthogonal direction on a pixel-by-pixel basis as follows: Di = −ln(So/Si)/(bi − bo), where, So and Si are signal intensities of each pixel at a diffusion sensitivity of bi and bo, respectively in any given direction i. The ADC was then calculated on a pixel-by-pixel basis by combining the three orthogonal diffusion coefficients as follows: D = [(Dx2 + Dy2 + Dz2)/3]1/2.
Once the ADC maps were constructed, regions outside the brain were removed through manual cropping of the skull and the dura. A histogram was then computed for each subject with a bin width of 0.6% of the maximum value of the ADC of 3.0 × 10−3 mm2/s. Histograms were then normalized across subjects in various groups for differences in brain size. The histograms were plotted and the peak locations, and the whole-brain average ADC were analyzed within each group. A t test between the control group and the patients in the four groups was computed for each of the parameters at a P < .05 level of significance.
Medical records were reviewed to determine the time of MR imaging study after admission and the average GCS scores at admission and discharge for individual groups. GCS scores of each group were correlated with their corresponding peak and mean ADC values.
Results
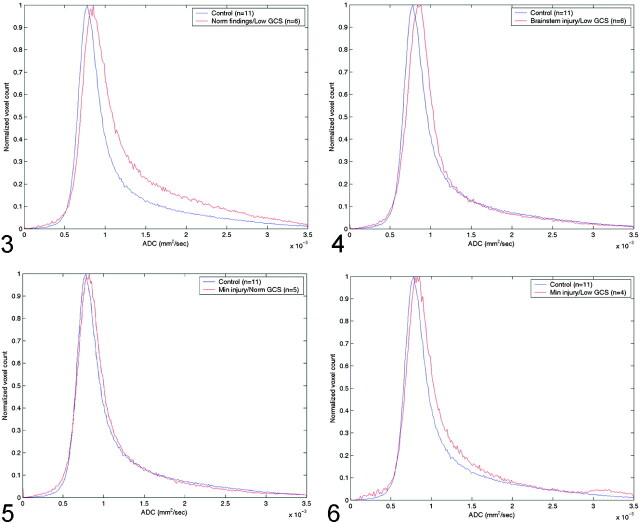
The Table shows the peak and average whole-brain ADC values, the average time from admission to MR imaging study, the average GCS score on admission and discharge, and the P values for the t test in the four groups of patients with TBI. Figure 3 shows the differences in ADC histograms between the control group and group A patients. Maximum values of these histograms were significant different (P < .0019), with a significant skewness in group A patients. Figure 4 shows a similar pattern, albeit with less skewness when the control group was compared with group B patients (P < .00129). Group C patients also had increased ADC values, as shown in Figure 5 but the difference was not statistically significant (P < .126). However, peak ADC values were significantly different between control subjects and group D (P < .0148), as shown in Figure 6. Average ADC significantly differed from control values in only group A (P < .013). For each group, peak ADC was strongly correlated with GCS score (R2 = 0.67). No strong correlation was observed between GCS scores and average ADCs among the patients (R2 = 0.041).
ADC values, GCS scores, and t test P values
| Value | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| Mean ADC | 1.33 ± 0.17 | 1.15 ± 0.13 | 1.08 ± 0.75 | 1.21 ± 0.18 |
| Peak ADC | 0.865 ± 0.06 | 0.840 ± 0.03 | 0.804 ± 0.03 | 0.831 ± 0.39 |
| P value, t test vs control | ||||
| Mean ADC | .013 | .49 | .143 | .23 |
| Peak ADC | .0019 | .00129 | .126 | .0148 |
| Age, years | 46 ± 26.4 | 25 ± 12.15 | 36 ± 18.9 | 47 ± 24.4 |
| GCS score | ||||
| Admission | 8 (6–10) | 6 (3–10) | 15 | 6 (3–10) |
| Discharge | 9 (7–11) | 11 (10–14) | 15 | Not available |
| Time of MR imaging after injury, days | 4 (1–10) | 8 (1–18) | 3 (1–9) | 9 (1–23) |
Note.—For the control group, mean ADC = 1.15 ± 0.12, and peak ADC = 0.785 ± 0.03. Other than P values, data are the mean ± SD or the mean (range).
Fig 3.
Comparison of normal whole-brain ADC histograms with histograms in patients with normal MR findings but a low GCS score (group A).
Fig 4.
Comparison of normal whole-brain ADC histograms with histograms in patients with brain-stem injuries and a low GCS score (group B).
Fig 5.
Comparison of normal whole-brain ADC histograms with histograms in patients with minimal brain injury and a normal GCS score (group C).
Fig 6.
Comparison of normal whole-brain ADC histograms with histograms in patients with minimal brain injury and a low GCS score (group D).
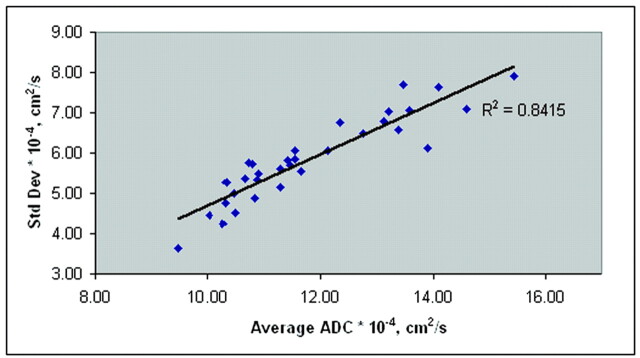
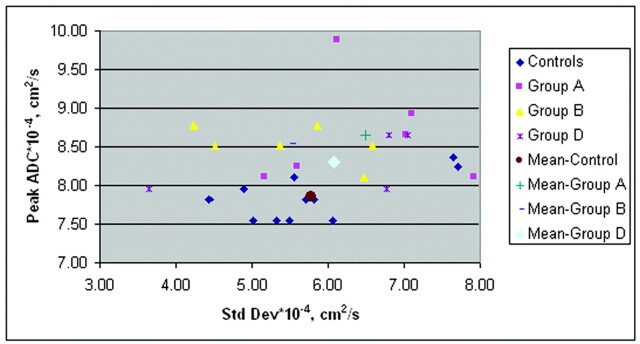
Figure 7 shows a plot of the SD variation with increasing mean ADC values from the control subjects and all patients. The increase in SD was highly correlated with an increase in the average whole-brain ADC (R2 = 0.84). Figure 8 shows the peak diffusion data from all control subjects and the patients with significantly different data (groups A, B, and D).
Fig 7.
Average ADCs of all control subjects and patients. SD of the mean ADC increases with increasing mean ADC, suggesting a heterogeneous distribution of ADC values in the injured brain.
Fig 8.
Peak ADCs of control subjects and patients and their associated SDs.
Discussion
In animal models of TBI, the nature of ADC changes varied depending on the type of head-injury model, the nature of TBI created, the interval between MR imaging and the primary insult, and the presence of hemorrhage in the lesion (9–12). Assaf et al (9) studied the temporal relationship between regional ADC values in acute and chronic traumatic brain lesions in the rat. ADC values measured at 2 and 24 hours after injury were significantly decreased when compared with values form the uninjured contralateral brain. However, values were significantly increased at 7 days after injury, when hypointensity seen on DW images correlated well with infarct areas on histologic specimens. Vorisek et al (10) studied diffusion parameters in rat cortex after stab wounds and reported that ADC values were altered not only near the injury but also in the entire ipsilateral hemisphere. These changes were attributed to astrogliosis, alterations in the extracellular volume fraction, and diffusion barriers in the injured cortex.
Decreased ADC values in both gray matter and white matter are reported in brain injury, with values in white matter significantly lower than those of gray matter (1, 14). Liu et al (1) obtained ADC values in diffuse axonal injury lesions in nine patients and compared them with values in white matter in healthy volunteers. A decreased ADC value was observed up to 18 days following initial injury and postulated to be a result of cytotoxic edema leading to restricted water diffusion in the intracellular compartment. Hemorrhage in the lesions may also have contributed to the restricted motion of water molecules. Other studies of conventional and DW-MR imaging in patients with diffuse axonal injury have shown decreased or increased ADC values (15, 16). Areas of increased diffusion were attributed to freely mobile water molecules in lesions with vasogenic edema and decreased diffusion to lesions with cytotoxic edema.
Unlike prior investigations, our preliminary study was performed to determine if whole-brain ADC values varied with the extent of TBI compared with values in control subjects. We also correlated these values with the altered levels of consciousness, as assessed by using the GCS. The GCS was initially described to enable consistent assessment of the depth and duration of altered consciousness and coma after TBI (17). On this scale, three aspects of behavioral response—motor response, verbal response, and eye opening—are independently evaluated. The GCS is widely accepted because it is practical and can be repeated frequently in a wide variety of clinical scenarios by medical staff without special training (17). Among the different criteria used in the first few days after TBI, the GCS is also helpful in predicting outcome after injury (16–19). In our study, GCS scores and whole-brain ADC values were significantly correlated. Significantly high whole-brain ADC values were observed in patients with low GCS scores. These preliminary results indicate that, like GCS scores, whole-brain ADC values are predictive of the severity of TBI and, possibly, outcome. A study with larger number of patients with varying degrees of TBI is necessary to confirm these findings.
In previous studies, regions of interest were used to determine ADC values in localized areas of injury (1, 14). However, we used whole-brain ADC values in patients with TBI, including cortical contusion and diffuse axonal shear injury. Whole-brain peak ADC values in all four groups were higher than those of control subjects. ADC values were significantly increased in patients with low GCS scores, conventional MR imaging evidence of brain stem injury, and normal conventional MR images (Group A). These results can be explained by less restricted diffusion in patients with TBI. Histologic correlation to determine the exact etiology of the ADC changes was not available in any of our patients. These high values may have resulted from a combination of vasogenic edema expanding the extracellular compartment and disruption of cell integrity, which decreased the restriction of movement of water molecules. We also observed a widened distribution of ADC values, as evidenced by the increased SD on histograms among patients with higher ADCs (Figs 7 and 8). This increased SD may indicate diffusion inhomogeneity in the brain, and it appears to scale with decreasesin GCS score. Our whole-brain peak ADC values in healthy subjects agree with previously published results (20–22). Patients with low GCS scores with conventional MR imaging evidence of brain stem injury (Group B) and normal conventional MR images (Group A) had persistently decreased GCS scores on discharge (Table). This finding supporting our postulation that permanent disruption of cell integrity correlates with a suboptimal outcome. Zhang et al (20) also reported similar results of significantly increased whole-brain ADCs in 24 boxers with chronic TBI, as compared with values in an age-matched control group; they also postulated similar mechanisms to explain these increased values.
In TBI, DW imaging is useful to demonstrate additional areas of injury not depicted on T2-weighted, gradient echo, and FLAIR images (16). In our study, a small subgroup of patients with low GCS score (≤ 10) and normal conventional MR images had significantly increased ADC values, which indicates occult TBI on conventional MR images. These findings are consistent with previous histopathologic findings in TBI, especially diffuse axonal injury. Even an experienced pathologist suspecting these injuries might visualize these lesions only by using special staining techniques on careful microscopic examination (23, 24). Some structural and physiologic derangements following brain trauma are apparently too small or possess signal intensity significantly different from that of adjacent normal adjacent tissue to be shown as being pathologic on conventional images. However, DW imaging is highly sensitive to changes in water diffusion in injured brain parenchyma and can demonstrate these subtle pathologic changes. In this study, follow-up MR imaging was not routinely performed to document further changes in ADC values. However, review of the medical records indicated that the average low GCS score remained abnormal, and in only one of six patients did the score improve to 14 before discharge.
Our study clearly shows an increase in the whole-brain ADCs compared with normal values. However, these results should be viewed in the context of their limitations. First, only a small number of patients with TBI were examined. Second, MR studies were performed 23 days after initial injury, and the ADC changes were correlated with only the admission GCS scores. No follow-up MR imaging or long-term clinical follow-up results were available to determine if ADC values were helpful in predicting neurologic outcomes. Third, it has been shown that advancing age is associated with a small but significant increase of water diffusivity in human white matter (21). This study showed that patients who were at least 60 years old had a significantly higher ADC than that of younger patients. Although we cannot completely rule out the effect of age on increasing ADCs, given the wide age range in each group, we expect the effect of age on the group ADCs to be minimal. A larger study of patients undergoing MR imaging during the subacute period (3–7 days) after head injury is likely to provide a more comprehensive explanation of the injury and recovery process. This work will require routine follow-up MR studies and clinical evaluation up to 3–6 months after TBI and may provide insight into the relationship between ADC changes and clinical outcomes. A larger study will also allow one to remove the effects of aging on the ADC values. Future studies should also include CSF-suppression techniques to achieve a more accurate assessment of ADC changes in the brain parenchyma.
Conclusion
The strong overall correlation between ADC values and GCS scores in this study leads us to believe that whole-brain diffusion values can be useful in assessing patients with TBI. In a small subgroup of such patients, whole-brain ADC values may be abnormal, even when conventional MR images are normal. Thus, DW-MR imaging increases the sensitivity of the MR imaging for the detection of TBI. Further studies with larger populations are needed to more precisely define the correlation of altered ADCs with types of TBI and the value of ADC changes in the whole brain or selected regions in predicting the long-term sequelae of TBI.
References
- 1.Liu AY, Maldjian A, Bagley LJ, Sinson GP, Grossman RI. Traumatic brain injury: diffusion-weighted MR imaging findings. AJNR Am J Neuroradiol 1999;20:1636–1641 [PMC free article] [PubMed] [Google Scholar]
- 2.Gean DA. Imaging of Head Trauma. New York: Raven Press;1994. :1–25
- 3.Wittsack H, Ritzl A, Wenserski F, et al. MR imaging in acute stroke: diffusion-weighted and perfusion imaging parameters for predicting infarct size. Radiology 2002;222:397–403 [DOI] [PubMed] [Google Scholar]
- 4.Sunshine JL, Tarr RW, Lanzieri CF, Landis DMD, et al. Hyperacute stroke: ultrafast MR imaging to triage patients prior to therapy. Radiology 1999;212:325–332 [DOI] [PubMed] [Google Scholar]
- 5.Moseley ME, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation T2- weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol 1990;11:423–429 [PMC free article] [PubMed] [Google Scholar]
- 6.Chien D, Kwong K, Gress D, Buonanno F, Buxton R, Rosen B. MR diffusion imaging of cerebral infarction in humans. AJNR Am J Neuroradiol 1996;13:1097–1102 [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher M. Diffusion and perfusion imaging for acute stroke. Surg Neurol 1995;43:606–609 [DOI] [PubMed] [Google Scholar]
- 8.Ito J, Marmarou A, Barzo P, Fatouros P, Corwin F. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J Neurosurg 1996;84:97–103 [DOI] [PubMed] [Google Scholar]
- 9.Assaf Y, Beit-Yannai E, Shohami E, Berman E, Cohen Y. Diffusion- and T2-weighted MRI of close-head injury in rats: a time course study and correlation with histology. Magn Reson Imaging 1997;15:77–85 [DOI] [PubMed] [Google Scholar]
- 10.Vorisek I, Hajek M, Tintera J, Nicolay K, Sykova E. Water ADC, extracellular space volume, and tortuosity in rat cortex after traumatic injury. Magn Reson Med 2002;48:994–1003 [DOI] [PubMed] [Google Scholar]
- 11.Alsop DC, Muria H, Detre JA, McIntosh TK, Smith DH. Detection of acute pathologic changes following experimental traumatic brain injury using diffusion-weighted magnetic resonance imaging. J Neurotrauma 1996;13:515–521 [DOI] [PubMed] [Google Scholar]
- 12.Hansstock CC, Faden AI, Benall MR, Vink R. Diffusion-weighted imaging differentiates ischemic tissue from traumatized tissue. Stroke 1994;25:843–848 [DOI] [PubMed] [Google Scholar]
- 13.Bartnik BL, Kendall EJ, Obenaus A. Cortical devascularization: quantitative diffusion weighted magnetic resonance imaging and histological findings. Brain Res 2001;915 ;133–142 [DOI] [PubMed] [Google Scholar]
- 14.Nakahara M, Ericson K, Bellander BM. Diffusion-weighted MR and apparent diffusion coefficient in the evaluation of severe brain injury. Acta Radiol 2001;42:365–369 [DOI] [PubMed] [Google Scholar]
- 15.Hergan K, Schaefer PW, Sorensen GA, Gonzalez RG, Husiman TAGM. Diffusion-weighted MRI in diffuse axonal injury of the brain. Eur Radiol 2002;12:2536–2541 [DOI] [PubMed] [Google Scholar]
- 16.Husiman TAGM, Sorensen AG, Hergan K, Gonzalez RG, Schaefer PW. Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head trauma. J Comput Assist Tomogr 2003;27:5–11 [DOI] [PubMed] [Google Scholar]
- 17.Teasdale G, Jennet B. Assessment of Coma and Impaired consciousness: a practical scale. Lancet 1974;13:81–84 [DOI] [PubMed] [Google Scholar]
- 18.Jennett B, Teasdale G, Braakman R, Minderhoud J, Kinll-Jones R. Predicting outcome in individual patients after severe head injury. Lancet 1976;15:1031–1034 [DOI] [PubMed] [Google Scholar]
- 19.Servadei F, Nasi MT, Cermonini AM, et al. Importance of a reliable admission Glasgow coma scale score for determining the need for evacuation of posttraumatic subdural hematomas: a prospective study of 65 patients. J Trauma 1998;44:868–873 [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Ravdin LD, Relkin N, et al. Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury. AJNR Am J Neuroradiol 2003;24:52–57 [PMC free article] [PubMed] [Google Scholar]
- 21.Engelter ST, Provenzale JM, Petrella JR, DeLong DM, MacFall JR. The effect of aging on the apparent diffusion coefficient of normal-appearing white matter. AJR Am J Roentgenol 2000;175;2:425–430 [DOI] [PubMed] [Google Scholar]
- 22.Carhuapoma JR, Barker PB, Hanley DF, Wang P, Beauchamp NJ. Human brain hemorrhage: quantification of perihematoma edema by use of diffusion-weighted MR imaging, AJNR Am J Neuroradiol 2002;23:1322–1326 [PMC free article] [PubMed] [Google Scholar]
- 23.Adams JH, Doyl D, Ford I, et al. Diffuse axonal injury in head injury: definition, diagnosis, and grading. Histopathology 1989;15:49–59 [DOI] [PubMed] [Google Scholar]
- 24.Strich S. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J Neurol Neurosurg Psychiatr 1956;19:163–185 [DOI] [PMC free article] [PubMed] [Google Scholar]