Abstract
BACKGROUND AND PURPOSE: Conformity between self-expanding Wallstents and vascular anatomy is limited. Because of a lack of longitudinal flexibility, straightening effects on vascular curves occur and may result in stent-induced kinking. Our purpose was to evaluate the conformity of self-expanding stents with the course and endoluminal surface of silicone models of the normal human carotid artery.
METHODS: Five different types of self-expanding carotid stents were implanted into simplified pulsatile perfused silicone models of the carotid bifurcation. The models embody elastic properties of the vessel wall similar to those of normal human arteries. All stents had the same nominal diameter and length and bridged the external carotid artery origin as well as a consecutive curve at the initial segment of the internal carotid artery. Conventional radiographs of the model were compared before and after stent placement to record changes of shape and course of the silicone artery. Dehiscences between stent filaments and arterial wall were measured on digital subtraction angiograms of the model.
RESULTS: Implantation of braided Wallstents or the Expander with continuous filaments induced considerable straightening effects on the bifurcation angle, as well as on the curves of the internal carotid artery. Segmented designs of modular nitinol stents complied better with vascular tortuosity and showed improved adaption between stent and the endoluminal surface of the model.
CONCLUSION: Model experiments show that segmented nitinol stents improve the conformity between the prosthesis and vascular anatomy, and confirm new carotid stent concepts as an alternative to the Wallstent.
Carotid angioplasty with stent placement has emerged as a potential alternative to carotid endarterectomy. In early carotid stent trials, balloon-expandable stents such as the Palmaz stent were used; however, follow-up studies showed cases with stent compression and consequent thrombosis or restenosis (1, 2). After these reports, self-expanding stents, such as the Wallstent, with intrinsic radial expansion forces and memory were increasingly used in the carotid artery (3).
In previous studies, we showed a limited conformity between self-expanding Wallstents and vascular anatomy. Straightening effects on vascular curves occur due to a lack of longitudinal flexibility of the stent and may induce kinks of tortuous arteries. Also, incomplete apposition between the stent-filaments and the arterial wall can be detected that potentially increases the risk of acute embolic complications (4). The purpose of our experimental study was to evaluate the conformity of four new self-expanding nitinol carotid stents with the course and endoluminal surface of silicone models of the normal human carotid artery and to compare the results with the performance of the carotid Wallstent.
Methods
The models were simplified elastic transparent silicone replicas (Elastrat, Geneva, Switzerland; Fig 1) of a cast of the human carotid bifurcation, which was derived from a cadaver by using dentistry prosthetic techniques. Liquid silicone was painted manually several times on wax copies of the arteries to create a model with elastic properties and wall thickness similar to that of a normal human carotid artery, which was determined in a previous series of measurements (5). The diameter of the common carotid artery (CCA) portion of the model was 8 mm, and the diameter of the initial segment of the internal carotid artery (ICA) was 6 mm. The proximal ICA segment showed a circumscribed curve that counterbalanced the initial angulation of 30° from the course of the CCA. The model was fixed in an acrylic box with the maximal opening of the bifurcation in the anteroposterior direction and connected to an artificial circulation. It was free floating in the acrylic box and was not embedded in a tissue equivalent. The temperature of the circulating water in the pulsatile perfused model was adjusted to 37°C.
Fig 1.
Simplified silicone model of the human carotid artery bifurcation with a nominal angulation between the CCA and initial ICA segment of 30° counterbalanced by a curve.
Five currently available self-expanding carotid stents were evaluated in our model (Table 1). Three stents (Jostent Self-X, Jomed, Beringen, Switzerland; SMART-Stent, Cordis, Miami, FL; Zilver-Stent, Cook, Bjaeverskov, Denmark) are laser cut from a nitinol tube and composed from ringlike zigzag segments that are only partially bridged in the longitudinal direction to increase flexibility of the prosthesis. The Expander (Medicorps, Nancy, France) is knitted from a meshwork of continuous nitinol filaments similar to the design of the Wallstent (Schneider-Boston-Scientific, Galway, Ireland) with its meshwork from a cobalt-chrome alloy. All stents had the same nominal diameter of 8 mm and a length of 60 mm after deployment.
TABLE 1:
Self-expanding carotid stent used for model experiments
| Stent | Material | Design |
|---|---|---|
| Carotid Wallstent | Cobalt-chrome alloy | Braided |
| Expander | Nitinol | Braided |
| Jostent Self-X | Nitinol | Segmented |
| SMART-Stent | Nitinol | Segmented |
| Zilver-Stent | Nitinol | Segmented |
Under fluoroscopic guidance in the digital subtraction angiography (DSA) unit, they were implanted into the silicone bifurcations through a 7.5F introducer sheath and a 7F guiding catheter. All stents completely bridged the external carotid artery origin and the curve of the initial ICA segment (Fig 2). Postdeployment angioplasty was not performed in this experiment.
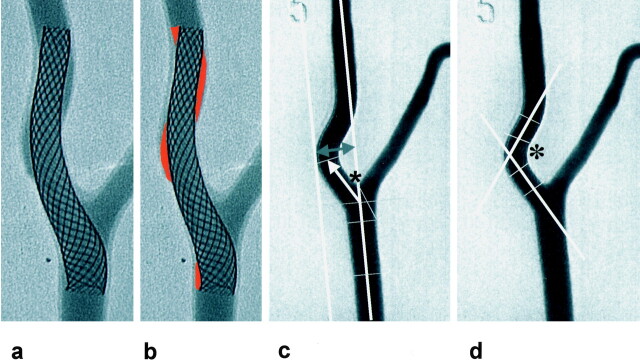
Fig 2.
Digital images of the model after implantation of a self-expanding carotid stent covering the ICA curve and the bifurcation as a basis for the measurement of parameters for quantification of stent conformity.
A and B, The dehiscence factor is calculated by dividing the areas of dehiscence (highlighted in B) by the area covered by the stent.
C, Measurement of the ICA offset as the maximal deviation of ICA tortuosity perpendicular to the CCA midaxis (upper arrows). Definition of the CCA-ICA angle as the angulation (asterisk) between the CCA midaxis and the midaxis of the initial ICA segment up to a level of 1.5 cm above a bifurcational 0-level is indicated by a line through the midpoint of the external carotid artery origin.
D, Measurement of the ICA angle (asterisk) between the tangents through the midaxes of both limbs of the ICA curve.
To evaluate the conformity of the stents with the course and endoluminal surface of the silicone models, conventional radiographs of the model in the same posteroanterior projections were compared before and after stent placement to record changes of shape and course of the silicone artery. To quantify morphologic changes induced by the stent, three parameters were defined and measured: 1) To determine areas with a lack of stent apposition, the dehiscence ratio was defined as the area between stent filaments and vessel wall in relationship to the area within the stent lumen. We put enlarged lateral images of the DSA image after stent placement on graph paper with a grid of 1-mm intervals and manually measured the area. 2) Extensions of the tortuous ICA perpendicular to the extended axis of the CCA were defined as ICA offset, and the ratio of pre–and post–stent placement values was calculated. 3) The angle between the midaxes of the CCA and the initial ICA segment up to a level of 1.5 cm distal to the bifurcation was measured according to previously published proposals (4). In addition, the opening angle of the ICA curve was measured, as shown in Fig 2. Average results of two implantation procedures for each stent were chosen for evaluation.
Results
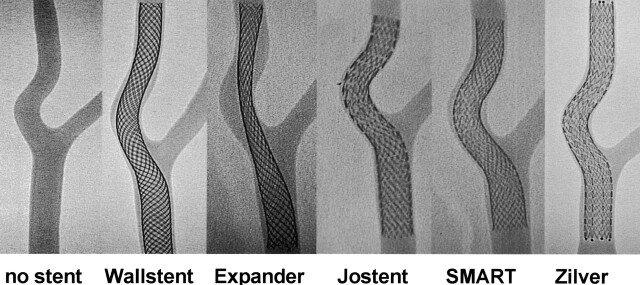
Conventional radiographic results after implantation of five kinds of carotid stent into the models are demonstrated in Fig 3. Results of dehiscence ratio, offset ratio, and CCA-ICA angle and ICA angle measurements on DSA images are shown in Table 2.
Fig 3.
Digital radiographs of the model without stent and after implantation of five types of self-expanding carotid stents. Note the differences of stent conformity between stents braided from continuous filaments (Wallstent and Expander) and segmented nitinol stents with an open cell design (Jostent, SMART and Zilver). See text for further explanations.
TABLE 2:
Comparison of stent conformity: average results of measurements
| Stent Type | Dehiscence Ratio | ICA Offset (mm) | CCA-ICA Angle | ICA Angle |
|---|---|---|---|---|
| No stent | — | 16 | 38° | 111° |
| Wallstent | 0.09 | 12 | 21° | 145° |
| Expander | 0.35 | 11 | 20° | 138° |
| Jostent | 0 | 14 | 31° | 112° |
| SMART | 0.01 | 13 | 29° | 117° |
| Zilver | 0 | 14 | 30° | 111° |
Visual evaluation proved a lack of apposition mainly with the use of the Wallstent and especially the Expander, whereas the three types of segmented nitinol stents showed no major dehiscences between stent struts and the vessel wall of the model. The difference in the dehiscence ratios between the Wallstent and the Expander was substantial. In accordace with visual evaluation, the dehiscence ratios of the two braided stents with continous filaments were higher than those of the segmented nitinol stents, which were close to zero. The braided stents modified the course of the model vessel and straightened the angulation and circumscribed curve of the ICA, which was transformed into a larger C-shaped bow. The segmented nitinol stents widely preserved the geometry of the carotid bifurcation and the ICA curve. The Wallstent and the Expander clearly reduced the CCA-ICA angle as well as the ICA deviation (offset) perpendicular to the CCA axis, whereas the Jostent, SMART, and Zilver stents induced less reduction of the angle and minimal changes of the ICA offset. The ICA angle between both limbs of the ICA curve increased markedly with the use of braided stents with continous filaments. The segmented nitinol stents induced negliable widening of the ICA angle. The nitinol stents demonstrated the same geometric properties, and no major differences could be detected among the three different subtypes.
Discussion
Visual evaluation and measurement of geometric parameters after stent placement in a simplified silicone model of the human carotid bifurcation proved that geometric modification of the angulation and ICA tortuosity occur predominantly with the use of stents braided from continous longitudinal filaments, such as the Wallstent or Expander. The reduction of the CCA-ICA angle and ICA offset and the widening of the ICA curve occur due to straightening of the segment with stent, which also has been observed in clincal Wallstent cases (3, 4). The new segmented nitinol stents with an open cell design showed improved conformity with the vascular anatomy of the model and preserved the angulation between CCA and initial ICA segments as well as the ICA curve.
Several potential limitations to our experiment should be mentioned. First, our models embody the elastic properties of the vessel wall similar to those of normal human arteries, and observations made in our models may not translate to those in diseased arteries. Second, our models are free floating in the acrylic box and are not embedded in a tissue equivalent. Furthermore, our experiments differ substantially from the way of carotid stent placement in many instances in that 1) primary stent placement was always employed, 2) no post–stent placement angioplasty was performed, 3) 60-mm-long stents were the only lengths used, and 4) only a single geometry of the carotid bifurcations was studied.
Despite limitations of the model, these results correspond well with clinical experiences. Phatouros et al (6) discussed the superior “conformability” of the shape-memory-alloy-recoverable-technology (SMART) stent to the native vessel. In their preliminary experience with the SMART stent, they found that this stent is better able to adapt to the native vessel contour than are stents with a braided design, such as Wallstents, because the segmented geometric design of the SMART stent results in individual stent hoops behaving fairly independently of each other. They point out that stent geometry is an important factor for confomability of the self-expanding carotid stents.
Unlike the SMART stent, the Wallstent has a braided geometry in a tubular mesh configuration. The wires continue along the entire length of the stent and are not independent from each other. This limits longitunal flexibilty and especially the apposition between the stent struts and the vessel wall. Dehiscences between the stent and vessel wall of the model were only observed after implantation of braided mesh stents. Proponents of the Wallstent argue that a straightening effect and consequent reduction of tortousity is desirable to improve hemodynamics by reduction of irregular flow patterns. However, complications related with such straighening effects such as stent-induced kinking may occur (4, 7).
Up to now, published data are lacking that determine whether straightening of the bifurcation or preservation of vascular anatomy with the use of segmented nitinol stents is superior for the vascular or clincal outcome after carotid stent placement. Suboptimal recanalization results with the Wallstent and the frequent lack of stent apposition seem to have no major influence on clinical outcome and restenosis rate (7, 8). Mukherjee et al (9) compared the short- and intermediate-term results of carotid stent placement by using either nitinol or stainless-steel self-expanding stents in 178 patients. In their series, 89 patients received Wallstents and the other 89 patients were treated with SMART stents. Stroke rate and neurologic outcome were similar in both groups, and the clinical superiority of the new segmented nitinol carotid stent remains controversial.
Our model experiments suggest that improved conformity of the segmented nitinol stent may improve recanalization results in cases with tortuous anatomy or abrupt changes of the vessel diameter. However, concerns have been expressed that separation between the stent segments with subsequent plaque protrusion might occur, and that such discontinuity between the stent modules might induce irregular flow patterns. We should mention, however, the well-known problems with the segmented nitinol stents in curved vessels in which prongs or segments protrude into the vessel. This supplies not only a potential source of emboli, but also a source for other devices such as guidewires or embolic protection umbrellas to get hung up on the stent during withdrawal. Furthermore, there are some reports on the nitinol stents, particularly on the SMART stent, of carotid expansion and shelf formation on the distal end of the stent owing to the increased radial force with nitinol as opposed to the alloy used in the Wallstent.
Consequently, a new generation of nitinol carotid stent with a closed cell design has been developed to minimize the risk of separation between stent modules. This rapid technical evolution underlines that the ideal carotid stent does not exist. However, today we have the choice between different stent designs to comply best with a given anatomic situation.
Footnotes
Presented at the 39th annual meeting of the American Society of Neuroradiology, Boston, 2001.
References
- 1.Mathur A, Dorros G, Iyer SS, Vitek JJ, Yadav SS, Roubin GS. Palmaz stent compression in patients following carotid artery stenting. Cathet Cardiovasc Diagn 1997;41:137–140 [DOI] [PubMed] [Google Scholar]
- 2.Rosenfield K, Schainfeld R, Pieczek A, Haley L, Isner JM. Restenosis of endovascular stents from stents compression. J Am Coll Cardiol 1997;29:328–338 [DOI] [PubMed] [Google Scholar]
- 3.Phatouros CC, Higashida RT, Malek AM, et al. Carotid artery stent placement for atherosclerotic disease: rationale, technique, and current status. Radiology 2000;217:26–41 [DOI] [PubMed] [Google Scholar]
- 4.Berkefeld J, Martin JB, Theron JG, et al. Complications of carotid angioplasty and stenting. Neurosurg Focus 1998;5:1–19 [DOI] [PubMed] [Google Scholar]
- 5.Gailloud P, Pray JR, Muster M, Piotin M, Fasel JHD, Rufenacht DA. An in vitro anatomic model of the human cerebral arteries with saccular arterial aneurysms. Surg Radiol Anat 1997;19:119–121 [PubMed] [Google Scholar]
- 6.Phatouros CC, Higashida RT, Malek AM, et al. Endovascular stenting for carotid artery stenosis: preliminary experience using the shape-memory-alloy-recoverable-technology (SMART) stent. AJNR AM J Neuroradiol 2000;21:732–738 [PMC free article] [PubMed] [Google Scholar]
- 7.Berkefeld J, Turowski B, Dietz A, et al. Recanalization results after carotid stent placement. AJNR Am J Neuroradiol 2002;23:113–120 [PMC free article] [PubMed] [Google Scholar]
- 8.Piamsomboon C, Roubin GS, Liu MW, et al. Relationship between oversizing of self-expanding stents and late loss index in carotid stenting. Cathet Cardiovasc Diagn 1998;45:139–143 [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee D, Kalahasti V, Roffi M, et al. Self-expanding stents for carotid interventions: comparison of nitinol versus stainless-steel stents. J Invasive Cardiol 2001;13:732–735 [PubMed] [Google Scholar]