Abstract
OBJECTIVES:
To examine the association between central nervous system (CNS) medication dosage burden and risk of serious falls, including hip fractures, in individuals with a history of a recent fall.
DESIGN:
Nested case–control study.
SETTING:
Veterans Health Administration (VHA) Community Living Centers (CLCs).
PARTICIPANTS:
CLC residents aged 65 and older with a history of a fall or hip fracture in the year before a CLC admission between July 1, 2005, and June 30, 2009. Each case (n = 316) was matched to four controls (n = 1264) on age, sex, and length of stay.
MEASUREMENTS:
Outcomes were serious falls identified using International Classification of Diseases, Ninth Revision (ACD-9) or Current Procedural Terminology (CPT) E codes, diagnosis codes, or procedure codes associated with a VHA emergency department visit or hospitalization during the CLC stay. Bar code medication administration data were used to calculate CNS standardized daily doses (SDDs) for opioid and benzodiazepine receptor agonists, some antidepressants, antiepileptics, and antipsychotics received in the 6 days before the outcome date by dividing residents’ actual CNS daily doses by the minimum effective geriatric daily doses and adding the results. Multivariable conditional logistic regression models were used to evaluate the association between total CNS medication dosage burden, categorized as 0, 1 to 2, and 3 or more SDDs, and the outcome of recurrent serious falls.
RESULTS:
More cases (44.3%) than controls (35.8%) received 3.0 or more CNS SDDs (p = .02). Risk of serious falls was greater in residents with 3.0 or more SDDs than in those with 0 (adjusted odds ratio (aOR)=1.49, 95% confidence interval (CI)=1.03–2.14). Those with 1.0 to 2.9 SDDs had a risk similar to that of those with 0 SDDs (aOR=1.03, 95%CI=0.72–1.48).
CONCLUSION:
Nursing home residents with a history of a fall or hip fracture receiving 3.0 or more CNS SDDs were more likely to have a recurrent serious fall than those taking no CNS medications. Interventions targeting this vulnerable population may help reduce serious falls.
Keywords: older, central nervous system agents, falls
Falls occur in 10% to 13% of older nursing home (NH) residents.1 As many as 10% of these falls may be serious (e.g., resulting in hip fracture) and can result in loss of independence, an increase in activity of daily living disability, need for a higher level of skilled nursing care, and greater use of physical restraints.2 Leading contributors to falls in the NH include a history of falls, cognitive impairment, depression, and central nervous system (CNS) medications (opioid and benzodiazepine receptor agonists, some antidepressants, antiepileptics, antipsychotics).3,4
There are several sets of explicit criteria for potentially inappropriate prescribing in older adults (e.g., American Geriatric Society Beers criteria, Screening Tool of Older People’s Prescriptions (STOPP)) that recommend in that individuals with a recent history of falls avoid these CNS medications,5,6 yet our group published a study showing that more than two-thirds of NH residents with a history of a fall received an interacting CNS medication.7 In addition, the Beers and STOPP criteria are not intended to provide guidance for prescribers for individuals who have a clinical indication for a CNS medication. Ideally, determining a threshold at which combined CNS medication dosage or burden incurs the greatest risk of falls would help supplement current guidelines on their use in older adults,8 but evidence to describe a dose-dependent risk in NH residents is limited.9–13 A recent study found a limit above which CNS medication burden, defined using standardized daily doses (SDDs), was associated with greater risk of serious falls in Medicare-enrolled NH residents with a history of falls or fractures.9 That study had several limitations. Namely, medication administration data were not available, so it is not known whether participants received the medications. In addition, there were no data on benzodiazepine use because these medications were not covered under Medicare Part D at the time. Finally, the participant population was predominantly long-term care (length of stay > 90 days), and medication use may differ in individuals with short stays.9 With these factors in mind, the primary objective of this study was to examine the association between CNS medication burden and risk of serious falls and hip fracture in older Veterans Health Administration (VHA) Community Living Center (CLC) residents with a history of a fall or hip fracture.
METHODS
Research Design, Data Sources, and Sample
This was a nested case–control study of residents aged 65 and older with a history of a fall or hip fracture who were admitted to a VHA CLC between July 1, 2005, and June 30, 2009. The analysis dataset was constructed by linking Minimum Data Set (MDS) version 2.0, Veterans Affairs (VA) Medical SAS datasets, and Bar Code Medication Administration (BCMA) data. The VA Medical SAS datasets provide information on patient characteristics, diagnosis codes, and procedure codes associated with care for all inpatient and outpatient visits and procedures performed. BCMA data contain daily details on the medication administration process in the inpatient setting, which includes CLCs.
To define the sample, we identified residents with a full MDS version 2.0 admission assessment and admission date between July 1, 2005, and June 30, 2009, as recorded on this assessment. Next, we limited the cohort to those aged 65 and older on the date of admission to the VHA CLC and excluded residents who were comatose (MDS field B1) or bedridden (G6 modes of transfer, a = bedfast all or most of time). We then restricted the sample to those with a history of a fall or hip fracture. Using the VHA Medical SAS datasets, we identified those with a history of a fall (E code (880–888)) or hip fracture (ICD-9 diagnosis (820.x), ICD-9 procedure (7855, 7905, 7915, 7925, 7935, 7965), CPT (27227, 27228, 27230, 27232, 27234–27236, 27238, 27240, 27242, 27244–27246, 27248) codes) associated with a VHA emergency department (ED) visit or hospitalization within 365 days before the date of admission to the CLC. Individuals could also qualify if a history of a fall or hip fracture (fall within past 30 days, fall within the past 31–180 days, hip fracture within past 180 days) was noted on the MDS assessment. We further excluded residents receiving hospice care (MDS field PIAO = 1) and required them to have a 7-day minimum length of stay. This resulted in a final cohort of 24,869 residents. (See Supplementary Appendix S1 for flow chart of cohort construction.) Residents were followed until an outcome occurred, they were discharged from the CLC, 1 year after their date of admission, or the end of the study period (June 30, 2010), which-ever came first. Those with outcomes were defined as cases (N = 316). For each case, we randomly matched 4 controls who had a birthdate within 1 year of that of the case, had a length of stay of 7 days or longer on the date of the outcome, and were the same sex. We had to relax the age criterion from within 1 year to within 5 years for 8 cases (2.5%) to ensure that every case matched to at least 4 controls.
Outcome Measure
The outcome was a serious fall or a hip fracture associated with a VHA ED visit or hospitalization after admission to the CLC. Using previously published and validated approaches, ED visits and inpatient stays with the E codes, ICD-9 procedure (inpatient) or diagnosis codes, or CPT (outpatient) codes listed above were identified using the medical SAS datasets.14,15
Exposure
BCMA data were used to determine the CNS medications and doses that residents received within 6 days before the outcome date. We used this exposure period to ensure that any new CNS medication, with an average half-life of 24 to 36 hours, would be at a steady state concentration (4 half-lives) before the outcome occurred. We excluded medications on the outcome date because they could have been prescribed to treat a resident with a serious fall or hip fracture (e.g., opioids for pain).
We used BCMA data on the name of the medication, dose, dosage form (e.g., tablet, injection), order schedule (e.g., as needed, continuous), order dosage (e.g., 75 mg, 1 tablet, 25 mL), doses ordered (e.g., 1), doses given (e.g., 0), and date and time the medication was administered to determine the total daily dose for each CNS medication a resident received. The CNS medications included were from the following five classes: tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), or serotonin norepinephrine reuptake inhibitors (SNRIs); anti-epileptics; antipsychotics; benzodiazepine receptor agonists; and opioid receptor agonists. As described previously, the SDD for each CNS medication was calculated by dividing the resident’s actual daily dose, based on BCMA data, by the minimum effective geriatric daily dose (MEGDD).9,16 (See Supplementary Appendix S2 for the specific CNS medications and MEGDD.) We calculated the SDD for each prescription at the daily level and then added them together and divided by 6 to obtain the mean SDD over the 6 days before the outcome. Then, the total CNS medication burden (exposure variable) for each resident was determined using the following equation:
where “CNS drug” is the resident’s actual daily dose. (See Supplementary Appendix S3 for an example calculation of SDD.) 9,16 Similar to previous work,9,16 we created a categorical variable for CNS SDD (0, 1.0–2.9, ≥ 3.0) as the main exposure variable. For descriptive purposes only, we also created categorical variables for CNS medication use overall (yes/no), the 5 individual classes of CNS medications, and the number of CNS drug classes used (0, 1, 2, 3–5).
Covariates
We considered factors that can increase the risk of a serious fall or hip fracture;1,3,4,9 we included glucocorticoid medications because they have been shown to increase the risk of fractures in adults.17 The covariates from the MDS were age, sex, race and ethnicity, cognitive function, use of a walking aid, vision impairment, wandering, and urinary incontinence on admission to the CLC. Cognitive function was categorized as intact according to the Cognitive Performance Scale (score 0 or 1), mildly to moderately impaired (score 2, 3, 4), and severely impaired (score 5 or 6).18 MDS data and ICD-9 codes (332, 332.0, 332.1) from VHA Medical SAS datasets were used to determine the presence of Parkinson’s disease. We also calculated the Charlson Comorbidity Index, excluding dementia, using ICD-9 codes during the 12 months before admission based on a previously developed method.19 Using BCMA data, we created variables for the use of other medications on admission that may increase the risk of falls and fractures (other non–tricyclic antidepressants, SSRIs, and SNRIs; peripheral alpha blockers; skeletal muscle relaxants; oral glucocorticoids) and the total number of drugs after excluding the CNS medications of interest, the “other” medications listed previously, topical creams, multivitamins, laxatives, and antidiarrheals. Finally, we created variables for CNS medication indications (anxiety disorder, depression, seizure disorder, moderate to severe bodily pain) using data from the MDS and Medical SAS datasets and use of an acetylcholinesterase inhibitor as a marker for dementia using BCMA.
Statistical Methods
We summarized and compared resident characteristics and exposure measures of cases and controls using univariate conditional logistic regression models to account for matching. For the categorical exposure measures, we calculated frequencies and percentages for use of any CNS medication overall and for each class, number of classes of CNS medications, and CNS SDD categories. We calculated means with standard deviations and medians with interquartile ranges for the continuous dose measures of SDD overall and for each class. Finally, we developed multivariable conditional logistic regression models with CNS SDD (0, 1.0–2.9, ≥ 3.0) as the main exposure to evaluate the association between CNS medication burden and serious falls because these cut points were previously used in publications of Medicare NH residents and community-dwelling individuals.9,16 The adjusted model included the above-mentioned covariates, except the matching variables. Linear contrast was used to compare the effect of 3.0 or more with 1.0 to 2.9 SDDs. Post hoc sensitivity analyses were also performed using alternative approaches (0 vs < 1.0, 1.0–2.9, 3.0–3.9, ≥ 4; tertiles) to assess whether the risk was different at a different SDD. All analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC). The study was approved by the VA Pittsburgh Healthcare System institutional review board, and a waiver of consent was obtained.
RESULTS
The final analysis sample included 1,580 unique residents (316 cases, 1,264 controls) with a mean age of 80; 96.8% were male. Cases and controls were well matched for baseline characteristics except that cases were more likely to be white (86.7% v. 80.9%; p = .03) and have moderate or severe bodily pain (50.9% vs 43.0%; p = .02); controls were more likely to have vision impairment (35.3% vs 28.5%; p = .01) (Table 1).
Table 1.
Baseline Participant Characteristics
| Characteristic | Controls, n = 1264 | Cases, n = 316 | P-Value |
|---|---|---|---|
| Age, mean±SD | 80.1 (7.0) | 80.1 (7.1) | .96 |
| Age, n (%) | .84 | ||
| 65–74 | 315 (24.9) | 81 (25.6) | |
| 75–84 | 619 (49.0) | 149 (47.2) | |
| ≥85 | 330 (26.1) | 86 (27.2) | |
| Male, n (%) | 1224 (96.8) | 306 (96.8) | >.99 |
| Race, n (%) | .03 | ||
| White, non-Hispanic | 1022 (80.9) | 274 (86.7) | |
| Black, non-Hispanic | 124 (9.8) | 20 (6.3) | |
| Hispanic | 36 (2.8) | 11 (3.5) | |
| Missing, other | 82 (6.5) | 11 (3.5) | |
| Cognitive function (Cognitive Performance Scale score), n (%) | .10 | ||
| Intact (0 or 1) | 637 (50.4) | 170 (53.8) | |
| Mild to moderate impairment (2, 3, or 4) | 473 (37.4) | 121 (38.3) | |
| Severe impairment (5 or 6) | 154 (12.2) | 25 (7.9) | |
| Walking aid use, n (%) | 1130 (89.4) | 287 (90.8) | .46 |
| Vision impairment, n (%) | 446 (35.3) | 90 (28.5) | .02 |
| Wandering in last 7 days, n (%) | 123 (9.7) | 28 (8.9) | .64 |
| Parkinson’s disease, n (%) | 86 (6.8) | 17 (5.4) | .36 |
| Urinary incontinence, n (%) | 440 (34.8) | 92 (29.1) | .06 |
| Charlson Comorbidity Index (excluding dementia), mean±SD | 2.8 (2.5) | 3.2 (2.7) | .07 |
| Use of other medications that may increase risk of falls, fractures, syncope, n (%)a | 401 (31.7) | 106 (33.5) | .54 |
| Number of other medications that may increase the risk of falls, fractures, syncope, n (%) 1 | .49 | ||
| 0 | 863 (68.3) | 210 (66.5) | |
| 1 | 355 (28.1) | 90 (28.5) | |
| ≥2 | 46 (3.6) | 16 (5.1) | |
| Numbers of medications at admission excluding those above, n (%) 2 | .45 | ||
| 0 | 72 (5.7) | 14 (4.4) | |
| 1–3 | 355 (28.1) | 82 (25.9) | |
| 4–8 | 526 (41.6) | 130 (41.1) | |
| ≥9 | 311 (24.6) | 90 (28.5) | |
| Indication for CNS medications, n (%) | 791 (62.6) | 205 (64.9) | .45 |
| Anxiety, n (%) | 144 (11.4) | 34 (10.8) | .75 |
| Depression, n (%) | 105 (8.3) | 17 (5.4) | .08 |
| Seizure disorder, n (%) | 60 (4.7) | 15 (4.7) | 1.00 |
| Moderate to severe bodily pain, n (%) | 544 (43.0) | 161 (50.9) | .01 |
| Dementia medication, n (%) | 172 (13.6) | 35 (11.1) | .23 |
Non–tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, peripheral alpha-blockers, skeletal muscle relaxants, oral glucocorticoids.
Excluding antidepressants, antiepileptics, antipsychotics, benzodiazepine receptor agonists, opioid receptor agonists, peripheral alpha-blockers, skeletal muscle relaxants, acetylcholinesterase inhibitors, topical agents for the skin, multivitamins, laxatives, antidiarrheals.
SD=standard deviation.
Overall, 80.4% of cases and 77.4% of controls received a CNS medication within 6 days before an outcome (p = .24) (Table 2). Cases were more likely to receive a medication from 2 or more CNS classes (p = .009). There was no difference between the groups in percentage who received a drug from the individual CNS medication classes, except that 51.9% of cases and 33.8% of controls received an opioid (p < .001).
Table 2.
Residents Receiving Central Nervous System (CNS) Medications Within 6 Days Before Outcome
| CNS Medication Class |
Controls, n = 1,264 |
Cases, n = 316 |
||
|---|---|---|---|---|
| n (%) | P-Value1 | |||
| Antidepressant (serotonin norepinephrine reuptake inhibitor, selective serotonin reuptake inhibitor, tricyclic antidepressant) | 481 (38.1) | 112 (35.4) | .39 | |
| Antiepileptic | 296 (23.4) | 74 (23.4) | >.99 | |
| Antipsychotic | 296 (23.4) | 84 (26.6) | .24 | |
| Benzodiazepine receptor agonist | 237 (18.8) | 70 (22.2) | .17 | |
| Opioid receptor agonist | 427 (33.8) | 164 (51.9) | <.001 | |
| Any CNS medication | 978 (77.4) | 254 (80.4) | .24 | |
| Number of classes of CNS medications | .009 | |||
| 0 | 286 (22.6) | 62 (19.6) | ||
| 1 | 472 (37.3) | 95 (30.1) | ||
| 2 | 306 (24.2) | 92 (29.1) | ||
| 3–5 | 200 (15.8) | 67 (21.2) | ||
Conditional logistic regression model.
When all CNS medications and doses a resident received were included, cases had a mean 4.41 and median 2.50 SDDs; controls had a mean 3.32 and median 1.91 SDDs (p = .002) (Table 3). In addition, a greater proportion of cases received 3.0 or more SDDs (44.3% vs 35.8%; p = .02) (Figure 1). Of the individual CNS medication classes, the median SDD for opioids was 0.11 for cases and 0 for controls (p < .001).
Table 3.
Central Nervous System (CNS) Standardized Daily Dose Within 6 Days Before Outcome According to Medication Class
| CNS Medication Class |
Controls, n = 1,264 |
Cases, n = 316 |
P-Value1 |
|---|---|---|---|
| Mean±Standard Deviation, Median (Interquartile Range) | |||
| Antidepressant (serotonin norepinephrine reuptake inhibitor, selective serotonin reuptake inhibitor, tricyclic antidepressant) | 0.88 ± 1.51, 0 (0–1.50) | 0.81 ± 1.36, 0 (0–1.58) | .46 |
| Antiepileptic | 0.25 ± 0.60, 0 (0–0) | 0.24 ± 0.56, 0 (0–0) | .78 |
| Antipsychotic | 0.74 ± 2.59, 0 (0–0) | 1.02 ± 4.62, 0 (0–0.25) | .16 |
| Benzodiazepine receptor agonist | 0.36 ± 1.47, 0 (0–0) | 0.34 ± 1.07, 0 (0–0) | .83 |
| Opioids receptor agonists | 1.08 ± 3.22, 0 (0–0.67) | 1.99 ± 5.13, 0.11 (0–2.0) | <.001 |
| All CNS medications | 3.32 ± 4.97, 1.91 (0.25–4.01) | 4.41 ± 7.25, 2.50 (0.50–5.75) | .002 |
Conditional logistic regression model
Figure 1.
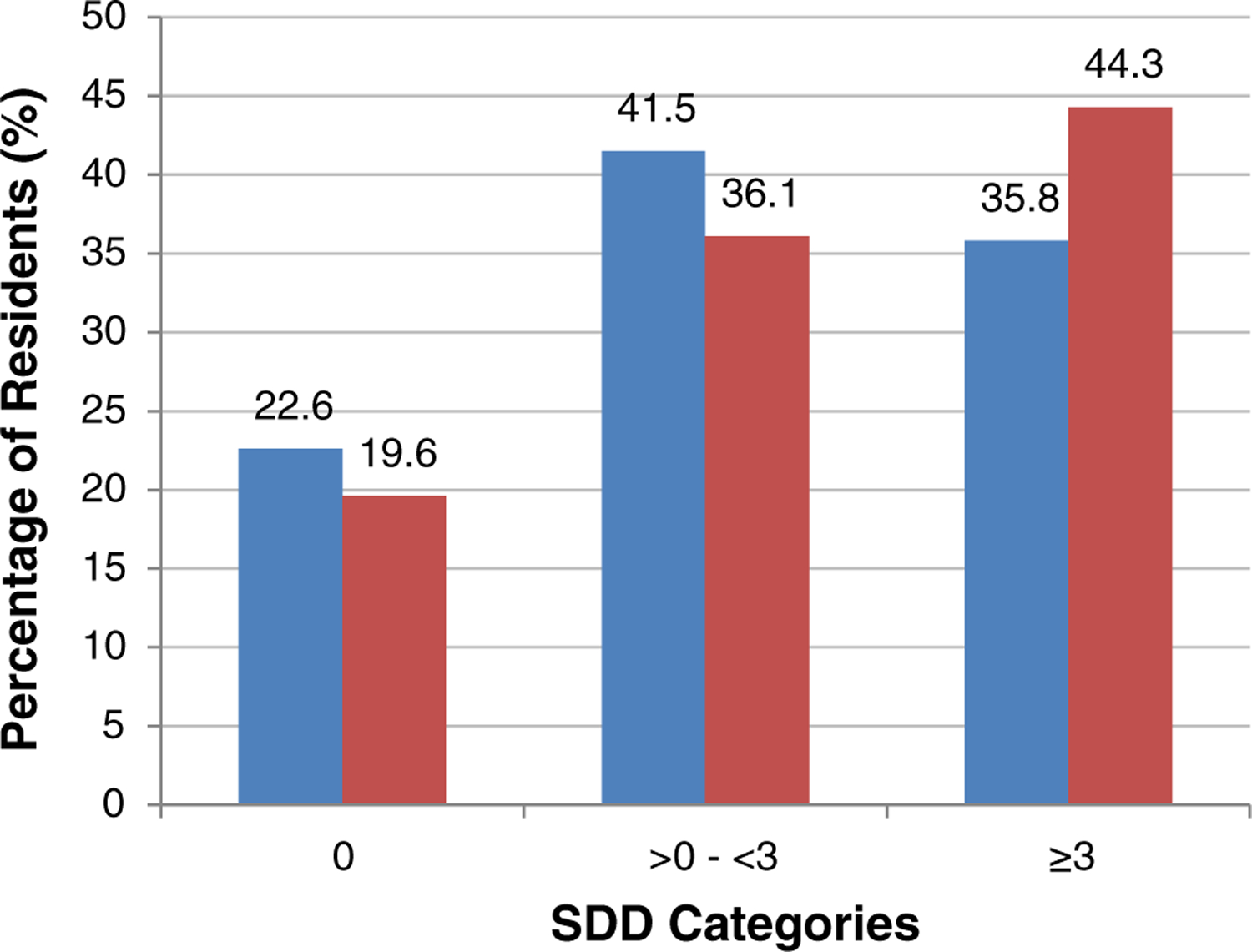
Residents in each central nervous system (CNS) standardized daily dose (SDD) category. Controls are blue bars; cases are red bars. [Color figure can be viewed at wileyonlinelibrary.com]
Risk of a serious fall was not significantly greater in residents who received a CNS medication SDD of 0.1 to 2.9 than in those who received none in the unadjusted and adjusted analyses (Table 4), although the odds of a serious fall were greater in residents who received 3.0 or more SDDs of CNS medications than in those who received none (odds ratio (OR)=1.46, 95% confidence interval (CI)=1.04–2.05; adjusted OR (aOR)=1.49, 95% CI=1.03–2.14) and in residents who received 3.0 or more SDDs of CNS medications than in those who received 0.1 to 2.9 (OR=1.45, 95% CI=1.09–1.93; aOR=1.44, 95% CI=1.07–1.93). The results remained unchanged when alternative cut points were explored as part of the sensitivity analysis (Supplementary Table S1).
Table 4.
Unadjusted and Adjusted Associations Between Standardized Daily Dose (SDD) of Central Nervous System (CNS) Medications and Serious Falls and Hip Fracture
| SDD |
Crude |
Adjusted1 |
|---|---|---|
| Odds Ratio (95% Confidence Interval) P-Value | ||
| 1–2 vs 0 | 1.00 (0.71–1.41) .99 | 1.03 (0.72–1.48) .86 |
| ≥3 vs 0 | 1.46 (1.04–2.05) .03 | 1.49 (1.03–2.14) .03 |
| ≥3 vs 1–2 | 1.45 (1.09–1.93) .01 | 1.44 (1.07–1.93) .02 |
Conditional logistic regression adjusted for race; ethnicity; cognitive function; walking aid use; vision impairment; wandering; Parkinson’s disease; urinary incontinence; Charlson Comorbidity Index (excluding dementia); other medications that may increase risk of falls, fracture, or syncope (non–serotonin norepinephrine reuptake inhibitor, selective serotonin reuptake inhibitor, or tricyclic antidepressants; peripheral alpha-blockers; skeletal muscle relaxants; oral glucocorticoids); number of medications at baseline (excluding antidepressants, antiepileptics, antipsychotics, benzodiazepine receptor agonists, opioid receptor agonists, peripheral alpha blockers, skeletal muscle relaxants, acetylcholinesterase inhibitors, topical agents for the skin, multivitamins, laxatives, antidiarrheals); and CNS medication indications (anxiety, depression, seizure disorder, moderate/severe, dementia medications)
DISCUSSION
This comprehensive, nested case–control study of older VA CLC residents nationwide who had a history of falls or hip fracture found that those receiving 3.0 or more SDDs of CNS medications had a slightly greater risk of a recurrent serious fall than those receiving fewer than 3.0 SDDs. Post hoc sensitivity analyses were confirmatory of the threshold effect, which was also seen in a non-VHA NH study.9 In addition, our results are largely consistent with other literature on CNS medication burden in older NH residents.10–12 An observational cohort study of NH residents with dementia evaluated the dose–response relationship between CNS medications and falls and found that the risk of falls was almost 3 times as high in those prescribed antipsychotics, sedatives and hypnotics, and antidepressants.10 Their study used defined daily doses, a World Health Organization term that does not take into account the lower doses recommended for older adults.13 Another observational cohort study of adults aged 70 and older living in residential aged care facilities in Australia that assessed the dose–response relationship between anticholinergic and sedative medications and falls using the Drug Burden Index (DBI) found almost twice the risk of falls in those with a high DBI.11 The DBI calculation attempted to account for lower geriatric doses by using the minimum registered dose from Australian prescribing information, although medication exposure was assessed at baseline and from records preceding the 12-month follow-up period, which does not allow for evaluation of nonadherence or changes in medications over time. In a small cohort of skilled nursing facility residents, the risk of falls was highest in those taking 2 or more CNS medications concurrently,12 although the medication information was pulled from monthly chart reviews, and daily psychotropic medication doses were not measured for a dose–response relationship.12
A study assessing CNS medication burden using SDDs in Medicare NH residents9 found almost twice the odds of a serious fall in residents receiving 3.0 or more CNS SDDs as in those not receiving any CNS medications, although benzodiazepine prescription information was not available, and the study relied on dispensing dates and days’ supply in Part D prescription fill records to infer SDDs received. By having access to the bar code medication administration data, we could accurately calculate CNS medication doses that residents took daily. Although both studies found an association between CNS medication burden and serious falls at a threshold of 3.0 or more SDDs, there were differences in medications received. A higher proportion of VA CLC cases received opioids, whereas a greater percentage of non-VHA NH cases received antidepressants. It is likely that differences in participant populations account for these findings. For example, more VA CLC residents reported severe pain; in contrast, non-VHA NH residents were more likely to have anxiety and depression.9 In addition, a higher proportion of VHA CLC residents are admitted for shorter stays, which may involve rehabilitation services or post-acute care recovery.20
Our results have important clinical implications. In individuals with a history of falls or fractures, alternative medications should be considered that may not be associated with risk (e.g., bupropion for depression instead of SSRIs),21 although it may be impossible to switch or stop CNS medications altogether in individuals who have no other options and are tolerating the drug. NH healthcare professionals now have another choice; they can consider decreasing doses of CNS medications so that the total SDD is less than 3.0. Older adults usually require lower doses of medications because of physiological changes, so there may be room to decrease the dose without compromising efficacy, although it is important to know how these adjustments affect the individual’s health and influence the risk of future falls or fractures.
Although our study addressed the most common limitation from previous studies (no data on actual medication administration), others remain. First, we may have underestimated the number of cases because E codes for falls appear to have been underused. We may also have missed a few outcomes that occurred in residents transferred to non-VA hospitals, but we do not believe this would result in a systematic bias. Also, most medications and doses are captured with BCMA, although some may have been missed (e.g., staff did not scan patient’s wrist band). Thus, the results from our study are probably conservative and biased toward null. Next, we did not have start dates for the medications, so we could not assess risk according to new versus chronic use, although we believe that this cohort of older adults receiving CNS medications—new and chronic—better represents the population in which healthcare providers are intervening. In addition, although we adjusted for multiple covariates, there may be other unknown confounders that would explain the association between CNS medication burden and serious falls. Finally, there is the question of generalizability because the data used in this study are older and may not reflect current prescribing practices if CNS medication use has decreased (fewer individuals with a CNS medication burden of ≥3.0) as a result of newer guidelines such as the Beers and STOPP criteria,5,6 although a systematic review from 2016 assessed potentially inappropriate medications (PIMs) in NH residents (a majority of which are CNS drugs) and found an increase in the proportion receiving PIMs in studies published after 2005 (49.8%) than in those from 1990 to 1999 (30.3%).22 In studies published in 2014 and 2015, the prevalence of at least 1 PIM ranged from 51.4% to 70.6% of NH residents.22 Also, although opioid use is decreasing in outpatients, opioids are still commonly part of postsurgical rehabilitation pain management, and older veterans are admitted to CLCs for rehabilitation. Therefore, we believe our results remain applicable in a sizable proportion of this population.
CONCLUSION
Older VA CLC residents with a history of falls or hip fracture receiving 3.0 or more CNS medication SDDs were more likely to have another serious fall than those taking no CNS medications. Interventions are needed to target this vulnerable population and evaluate whether decreasing the CNS medication burden below 3.0 SDDs reduces the risk of future serious falls.
Supplementary Material
Appendix S1. Flow Chart of Cohort Construction
Appendix S2. Central Nervous System Medications Taken by Nursing Home Residents and Minimum Effective Geriatric Daily Dose
Appendix S3. Example Calculation of CNS Medication Burden as Standardized Daily Dose (SDD)
Table S1: Sensitivity Analyses of Standardized Daily Dose (SDD) Cut-Points.
IMPACT STATEMENT.
We certify that this work is confirmatory of recent novel clinical research (Hanlon JT, Zhao X, Naples JG, et al. Central nervous system (CNS) medication burden and serious falls in older nursing home (NH) residents. J Am Geriatr Soc 2017; 65: 1183–1189), which found a limit above which CNS medication burden, defined using standardized daily doses, was associated with greater risk of serious falls in Medicare enrolled NH residents, although medication administration data were not available, so it is not known whether participants received the medications. In addition, no data on benzodiazepine use were available. The current study addresses both of these limitations in a veteran population. The potential effect of this research on clinical care includes interventions to target this vulnerable population and evaluate whether decreasing CNS medication burden reduces the risk of future serious falls.
ACKNOWLEDGMENTS
Financial Disclosure: This work was supported in part by National Institute of Aging Grants P30AG024827, Agency for Health Research and Quality Grant R18 HS023779, a Donoghue Foundation grant, and VA Health Services Research and Development Service Merit Awards IIR 14–297, IIR 14–306, IIR 15–115. Dr. Springer is funded by a Postdoctoral Fellowship through the VA Office of Academic Affairs.
Sponsor’s Role: None.
Footnotes
The paper was submitted as an abstract for the American Public Health Association Annual Meeting & Expo, November 10–14, 2018.
The views expressed in this paper are those of the authors, and no official endorsement by the Department of Veterans Affairs or the U.S. government is intended or should be inferred.
Conflict of Interest: Dr. Semla’s spouse is an employee of AbbVie and owns stock in AbbVie and Abbott.
REFERENCES
- 1.Quigley PA, Campbell RR, Bulat T, Olney RL, Buerhaus P, Needleman J. Incidence and cost of serious fall-related injuries in nursing homes. Clin Nurs Res 2012;21:10–23. [DOI] [PubMed] [Google Scholar]
- 2.Becker C, Rapp K. Fall prevention in nursing homes. Clin Geriatr Med 2010;26:693–704. [DOI] [PubMed] [Google Scholar]
- 3.Deandrea S, Bravi F, Turati F, Lucenteforte E, la Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatrics 2013;56:407–415. [DOI] [PubMed] [Google Scholar]
- 4.Seppala LJ, Wermelink AMAT, de Vries M, et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: II. Psychotropics. J Am Med Dir Assoc 2018;19:371.e11–371.e17. [DOI] [PubMed] [Google Scholar]
- 5.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63:2227–2246. [DOI] [PubMed] [Google Scholar]
- 6.O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2015; 44:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aspinall SL, Zhao X, Semla TP, et al. , the Veterans Affairs Community Living Center Pharmacotherapy Research Group. Epidemiology of drug-disease interactions in older veteran nursing home residents. J Am Geriatr Soc 2015;63:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aspinall SL, Hanlon JT, Niznik JD, et al. Deprescribing in older nursing home patients: Focus on innovative composite measures for dosage deintensification. Innovat Aging 2017;1:000–000. 10.1093/geroni/igx031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanlon JT, Zhao X, Naples JG, et al. Central nervous system medication burden and serious falls in older nursing home residents. J Am Geriatr Soc 2017;65:1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterke CS, van Beeck EF, van der Velde N, et al. New insights: Dose-response relationship between psychotropic drugs and falls: A study in nursing home residents with dementia. J Clin Pharmacol 2012;52:947–955. [DOI] [PubMed] [Google Scholar]
- 11.Wilson NM, Hilmer SN, March LM, et al. Associations between drug burden index and falls in older people in residential aged care. J Am Geriatr Soc 2011;59:875–880. [DOI] [PubMed] [Google Scholar]
- 12.Cooper JW, Freeman MH, Cook CL, Burfield AH. Assessment of psychotropic and psychoactive drug loads and falls in nursing facility residents. Consult Pharm 2007;22:483–489. [DOI] [PubMed] [Google Scholar]
- 13.Essential Medicines and Health Products: Defined Daily Dose (DDD) (online). Available at http://www.who.int/medicines/regulation/medicines-safety/toolkit_ddd/en/ Accessed March 1, 2018.
- 14.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol 1992;45:703–714. [DOI] [PubMed] [Google Scholar]
- 15.Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M. Using medical service claims to assess injuries in the elderly: Sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol 2000;53: 183–194. [DOI] [PubMed] [Google Scholar]
- 16.Hanlon JT, Boudreau RM, Roumani YF, et al. Number and dosage of central nervous system medications on recurrent falls in community elders: The Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci 2009;64A:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res 2000;15:993–1000. [DOI] [PubMed] [Google Scholar]
- 18.Hartmaier SL, Sloane PD, Guess HA, et al. Validation of the minimum data set cognitive performance scale: Agreement with the Mini-Mental State Examination. J Gerontol A Biol Sci Med Sci 1995;50A:M128–133. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45: 613–619. [DOI] [PubMed] [Google Scholar]
- 20.Williams BC, Fries BE, Mehr DR. Length of stay in VA nursing homes. Comparative characteristics of brief-, medium-, and long-stay residents. J Aging Health 1993:5:208–228. [DOI] [PubMed] [Google Scholar]
- 21.Hanlon JT, Semla TP, Schmader KE. Alternative medications for 2015 National Committee for Quality Assurance’s Healthcare Effectiveness Data and Information Set criteria for high-risk medications and potentially harmful drug-disease interactions in the elderly. J Am Geriatr Soc 2015;63: e8–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morin L, Laroche M, Texier G, Johnell K. Prevalence of potentially inappropriate medication use in older adults living in nursing homes: A systematic review. J Am Med Dir Assoc 2016;17:862.e1–862.e9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Flow Chart of Cohort Construction
Appendix S2. Central Nervous System Medications Taken by Nursing Home Residents and Minimum Effective Geriatric Daily Dose
Appendix S3. Example Calculation of CNS Medication Burden as Standardized Daily Dose (SDD)
Table S1: Sensitivity Analyses of Standardized Daily Dose (SDD) Cut-Points.


