Abstract
Summary: There are limited case reports of structural lesions causing Korsakoff syndrome. This report describes acute Korsakoff syndrome following localized, bilateral infarction of the mammillothalamic tracts (MTTs). Axial T2-weighted imaging revealed the lesions at the lateral wall level of the third ventricle and diffusion-weighted imaging confirmed that the left lesion was new and the right old. Korsakoff syndrome persisted 6 months after the onset. This case suggests that bilateral MTT dysfunction can lead to Korsakoff syndrome.
Since Korsakoff syndrome was first described in 1887, the syndrome has been associated with thiamine deficiency. Almost all of the cases described in the literature that were unrelated to alcoholism were related to malnutrition or malabsorption, including persistent emesis, intravenous feeding, gastrointestinal carcinoma, dialysis, and AIDS (1). There are limited case reports of structural lesions causing Korsakoff syndrome, although some causative tumors of the central nervous system or mass lesions have been described (2–5). Despite agreement on the neuropathologic process of this syndrome, the critical lesion sites for memory disorder have been debated. We present a case of Korsakoff syndrome following small infarctions involving the mammillothalamic tracts (MTTs) and consider the pathogenesis and structural involvement of Korsakoff syndrome by using conventional MR imaging.
Case Report
A 56-year-old right-handed diabetic man was brought to the emergency room because of persistent memory disturbance over the course of the previous 3 days. The patient was an executive of a nationwide agricultural cooperative who had no problems with cognition until this abrupt onset of the memory disturbance. At presentation, he could not remember recent conversations, events of the day, or where he had placed objects in his house. His conversation repeatedly contained confabulations. In addition to anterograde amnesia, he also had retrograde amnesia and could not recall events of the previous 4 years. He did not believe that his father had died, which occurred 2 years before this interview. Mental status testing revealed that he was alert and attentive but disoriented about month and year. The level of general intelligence, previously learned skills, immediate recall, and ability to calculate in short formulae were retained. Serial-sevens could be performed up to 86, at which point he lost concentration and stopped. Naming items, item recognition, repetitions, comprehension, reading, and writing were normal. The patient did not exhibit hemiparesis, hemisensory loss, ataxia, and nystagmus. CT scans and conventional MR images obtained 3 days after symptom onset showed small infarctions in the corpus callosum on the right, in the right caudate head, and on the right and left MTTs. The left MTT lesion was depicted by diffusion-weighted (DW) imaging and confirmed that it was new (Figs 1 and 2). The medial temporal regions were shown to be intact at CT and MR imaging (Fig 1). He was treated conservatively for cerebral infarctions associated with Korsakoff syndrome, and peroral aspirin was substituted for an intravenous antithrombin agent, which was the initial therapy. His diabetes mellitus was also treated intensively by a diabetiologist, but the patient’s amnesia persisted.
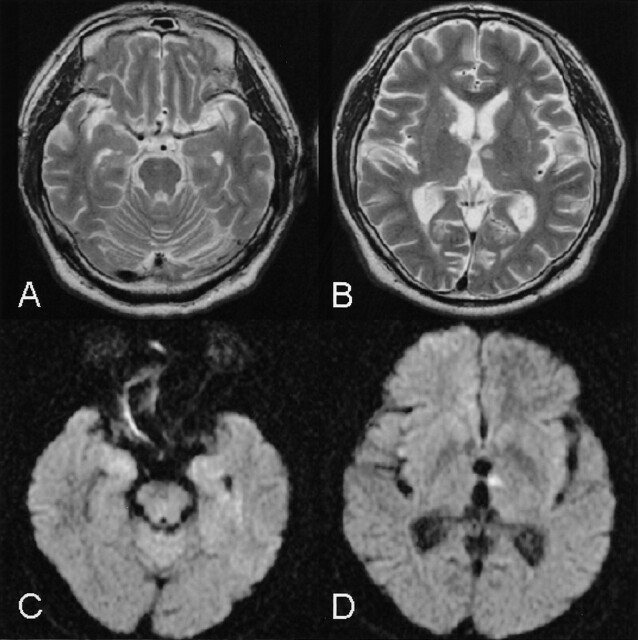
Fig 1.
T2-weighted images examined 3 days after onset (A and B) show multiple ischemic lesions in the midpons, the head of the right caudate nucleus, and the left anterior thalamus. A DW image confirms the left anterior thalamic lesion as a new ischemic lesion (D). Medial temporal lobes appear intact both on T2-weighted and DW images (A and C).
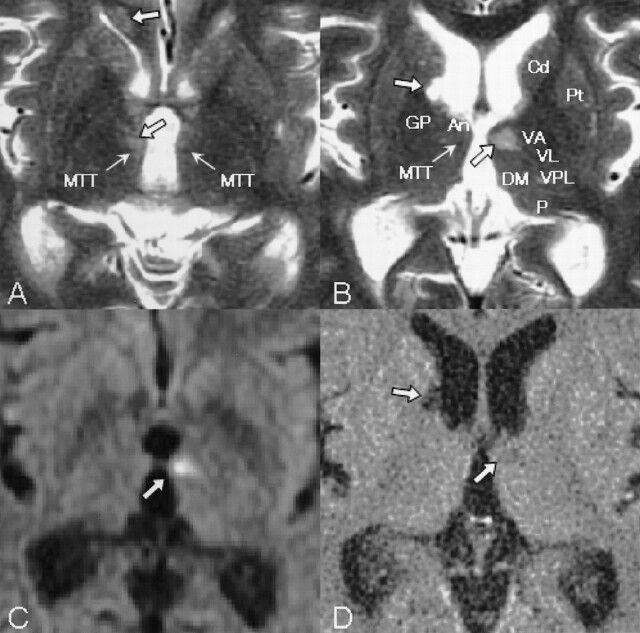
Fig 2.
Area of detail on T2-weighted images examined 3 days after onset (A and B) demonstrate anatomic structures, such as the anterior nucleus (An), caudate nucleus (Cd), dorsomedial nucleus (DM), globus pallidus (GP), MTT, pulvinar (P), putamen (Pt), ventral anterior nucleus (VA), ventral lateral nucleus (VL), and ventral posterolateral nucleus (VPL). An infarction on the left MTT (C, upswept thick arrow) demonstrated on a DW image is identical to that on the T2-weighted image (B, upswept thick arrow) and on the fluid-attenuated inversion recovery image, which was seen on a follow-up CT scan 4 weeks after onset (D, upswept thick arrow), which was the same as on MR images at admission. Older ischemic lesions were also seen in the corpus callosum on the right (A, upper descending thick arrow), on the right MTT (A, lower descending thick arrow), and in the head of the right caudate nucleus (B and D, descending thick arrow). Thick arrows show ischemic lesions, and thin arrows, MTTs.
Mental status testing 4 weeks after onset revealed no improvement of his memory. Serial-sevens could be performed to 79, although he gave up because of frustration. He still did not accept the fact that his father had died, although he said that he had noticed something odd about his memory. A follow-up CT scan showed that a small infarction of the left MTT was the same as that on previous images examined 4 weeks earlier.
Eight weeks after onset, his amnesia persisted. Mental status testing results were almost the same as those obtained 4 weeks after onset. Follow-up MR imaging 8 weeks after onset demonstrated small infarctions of both MTTs (Figs 3–5). The infarction over the right MTT was thought to be much older than the left, because the right MTT lesion was hypointense at DW imaging performed at admission and 8 weeks after onset. Six months after onset, his memory showed no improvement during a regular follow-up evaluation at the outpatient clinic.
Fig 3.
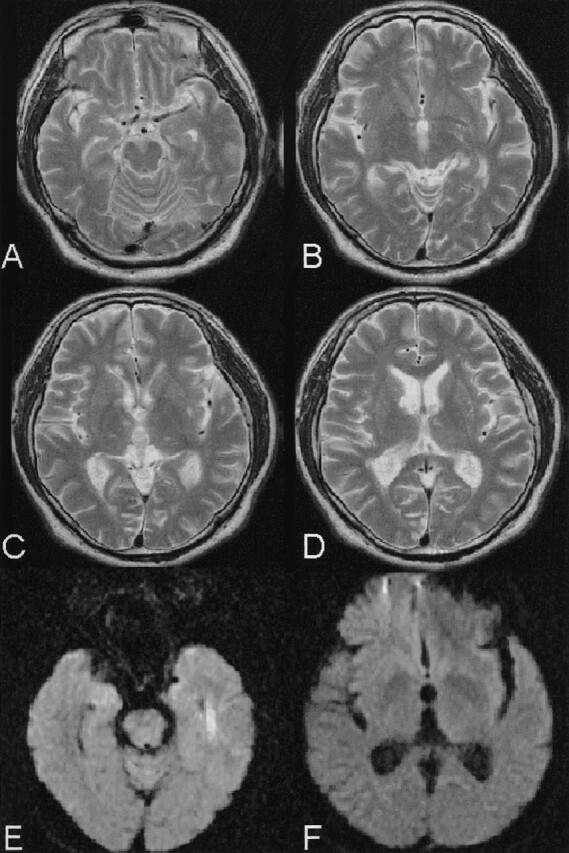
T2-weighted images obtained 8 weeks after onset show ischemic lesions in the corpus callosum on the right and the right anterior thalamus (C and D). The infarction of the left MTT (D) is identical to that on MR images obtained at admission (Fig 1). DW imaging shows no acute ischemic lesions (E and F). The medial temporal lobes (A and B) are intact, which is the same finding as that on the MR images obtained at admission (Fig 1).
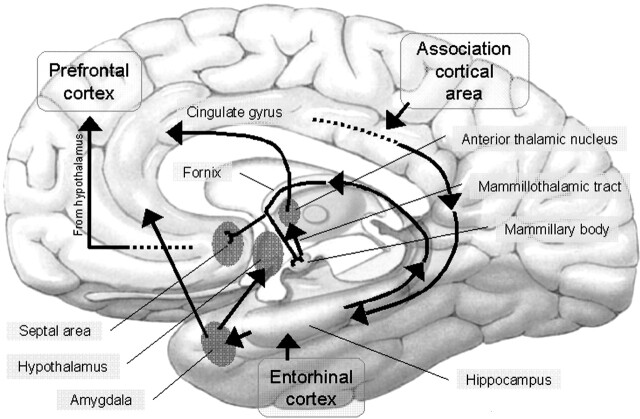
Fig 5.
Schematic figure of the limbic system and its connections. The hippocampus projects to the septal nuclei and the mammillary body via the fornix. The mammillary body projects to the anterior nucleus of the thalamus via the MTT. The anterior nucleus of the thalamus projects to the cingulated cortex via the internal capsule. The cingulated cortex projects back to the hippocampus via the cingulum bundle. Both MTT lesions can interrupt the input from the hippocampus, resulting in dysfunction of the neurocircuit in the limbic system.
Discussion
The clinical diagnosis of Korsakoff syndrome is based on the identification of an amnesic syndrome with a history of alcohol abuse or malnutrition. The diagnosis can be made when memory deficits are more prominent than other cognitive disorders in the domain of language, visuoperceptual functioning, problem solving, and judgment (2). The patient described in this report presented with a syndrome that mirrored Korsakoff syndrome: memory dysfunction with retrograde amnesia, anterograde amnesia, limited insight into dysfunction, and confabulation despite no history of chronic alcoholism or malnutrition.
The two above-mentioned findings—acute memory disorder identical to Korsakoff syndrome and MR imaging findings of bilateral MTT infarctions (the left lesion fresh and the right old)—indicate that the sites of involvement in acute Korsakoff syndrome are both MTTs, leading to bilateral MTT dysfunction.
Although there is agreement on the neuropathologic mechanisms of Korsakoff syndrome, the critical lesion sites for memory disorder have been debated. From the viewpoint of the critical lesion(s) for amnesic syndrome, the thalamus and the mammillary bodies are most commonly implicated (6). Victor et al (7) pointed out that all 24 patients in whom the medial-dorsal nucleus of the thalamus was affected had a clinical history of persistent memory impairment (Korsakoff syndrome). Mair et al (8) provided a careful pathologic and neuropsychological description of two patients with Korsakoff syndrome in whom autopsies showed lesions in the mammillary bodies and the midline and anterior portion of the thalamus but not in the medial-dorsal nuclei, which was closely replicated by Mayes et al (9). On the other hand, Heilman et al (10) reported Korsakoff syndrome resulting from bilateral fornix lesions.
Recent evidence suggests that the circuit involving the mammillary bodies, the MTT and the anterior thalamus rather than the medial dorsal nucleus of the thalamus, is particularly critical in the formation of new memories (6). Visser et al (11) showed anterograde amnesia in alcoholic Korsakoff syndrome is associated with atrophy of the nuclei in the midline of the thalamus, but not with atrophy of the mamillary bodies, the hippocampus, or the parahippocampal gyrus. In addition, Harding et al (12) concluded that neuronal loss in the anterior thalamic nuclei was found consistently only in alcoholic Korsakoff psychosis, although neurodegeneration of the hypothalamic mammillary nuclei and the mediodorsal thalamic nuclei was substantial in those with nonamnesic alcoholism and those with amnesic alcoholism with Wernicke encephalopathy.
From animal experiments, Mishkin (13, 14) argued that there are two limbic circuits in which combined lesions are required to produce severe amnesia: hippocampal (medial limbic) and amygdaloid (basolateral limbic) circuits. The anterior and dorsomedial thalamus and the medial temporal lobes are the anatomic locations (“nodal points”) wherein these two circuits converge and are, therefore, particularly vulnerable to the effect of discrete structural lesions (15–19). Consequently, lesions of both MTTs projecting to the anterior nucleus of the thalamus can affect the nodal points, resulting in a severe amnesic state (Fig 5).
Determining the contribution of an individual region in memory is difficult, because it is unusual to find anatomically circumscribed damage to only one structural region in a clinical setting. In our case, however, we encountered infarctions in both MTTs depicted by DW imaging. The left lesion was new, the right was old, and the medial temporal lobes (Fig 3) were normal. It is interesting to note that, from the viewpoint of the clinical course, the patient had no memory or cognitive disorder when he developed the right MTT lesion. We speculate that the right MTT lesion did not cause clinical symptoms. Figure 4 shows small lesions in the MTTs and demonstrates intact ventral anterior nucleus, ventral lateral nucleus, and ventral posterolateral nucleus on both sides. Thus, in this case, the pathogenetic candidate of acute Korsakoff syndrome was thought to be both the affected MTTs and the left MTT ischemia acted as a trigger of Korsakoff syndrome. Both MTT lesions may have cut off hippocampal input through the mammillary bodies, into the anterior nucleus, thereby producing a severe amnesic state.
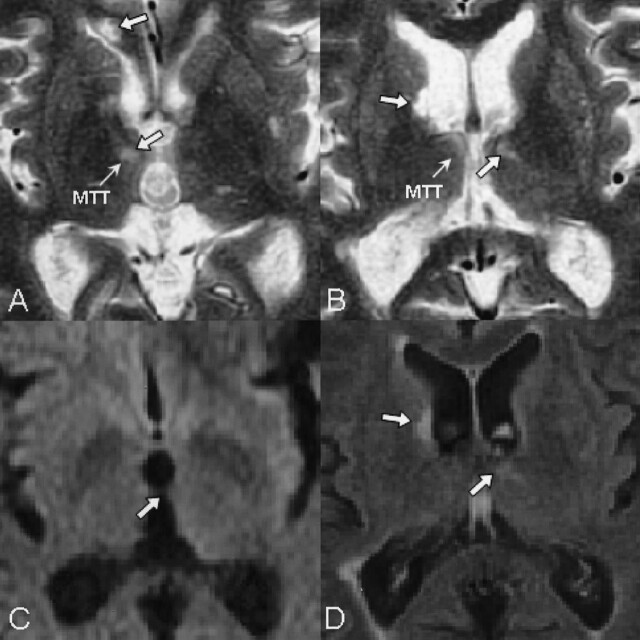
Fig 4.
Magnified T2-weighted images examined 8 weeks after onset demonstrate that both MTTs are affected (A and B), which are not visible on DW images (C). They are not acute ischemic lesions as shown on DW images (C) and on fluid attenuation inversion recovery images (D).
References
- 1.Shear PK, Sullivan EV, Lane B, Pfefferbaum A. Mammillary body and cerebellar shrinkage in chronic alcoholics with and without amnesia. Alcohol Clin Exp Res 1996;20:1489–1495 [DOI] [PubMed] [Google Scholar]
- 2.Toth C, Voll C, Macaulay R. Primary CNS lymphoma as a cause of Korsakoff syndrome. Surg Neurol 2002;57:41–45 [DOI] [PubMed] [Google Scholar]
- 3.Konovalov AN, Dobrokhotova TA, Voronina IA, Urakov SV. Case of Korsakoff syndrome and colloid cyst of the 3rd ventricle [in Russian] Zh Nevrol Psikhiatr Im S S Korsakova 1998;98:49–51 [PubMed] [Google Scholar]
- 4.Yarde WL, Kepes JJ, O’Boynick P. Craniopharyngioma presenting as Korsakoff psychosis. Kans Med 1995;96:22–23, 34 [PubMed] [Google Scholar]
- 5.Neciga EG, Peralta AG, Polaina M, et al. Cyst of the septum pellucidum and Korsakoff’s psychosis. Eur Neurol 1989;29:99–101 [DOI] [PubMed] [Google Scholar]
- 6.Kopelman MD. The Korsakoff syndrome. Br J Psychiatry 1995;166:154–173 [DOI] [PubMed] [Google Scholar]
- 7.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome: a clinical and pathological study of 245 patients, 82 with post-mortem examinations. Contemp Neurol Ser 1971;7:1–206 [PubMed] [Google Scholar]
- 8.Mair WG, Warrington EK, Weiskrantz L. Memory disorder in Korsakoff’s psychosis: a neuropathological and neuropsychological investigation of two cases. Brain 1979;102:749–783 [DOI] [PubMed] [Google Scholar]
- 9.Mayes AR, Meudell PR, Mann D, Pickering A. Location of lesions in Korsakoff’s syndrome: neuropsychological and neuropathological data on two patients. Cortex 1988;24:367–388 [DOI] [PubMed] [Google Scholar]
- 10.Heilman KM, Sypert GW. Korsakoff’s syndrome resulting from bilateral fornix lesions. Neurology 1977;27:490–493 [DOI] [PubMed] [Google Scholar]
- 11.Visser PJ, Krabbendam L, Verhey FR, et al. Brain correlates of memory dysfunction in alcoholic Korsakoff’s syndrome. J Neurol Neurosurg Psychiatry 1999;67:774–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 2000;123:141–154 [DOI] [PubMed] [Google Scholar]
- 13.Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature 1978;273:297–298 [DOI] [PubMed] [Google Scholar]
- 14.Mishkin M. A memory system in the monkey. Philos Trans R Soc Lond B Biol Sci 298:85–95 [DOI] [PubMed] [Google Scholar]
- 15.Speedie LJ, Heilman KM. Amnestic disturbance following infarction of the left dorsomedial nucleus of the thalamus. Neuropsychologia 1982;20:597–604 [DOI] [PubMed] [Google Scholar]
- 16.Markowitsch HJ. Can amnesia be caused by damage of a single brain structure? Cortex 1984;20:27–45 [DOI] [PubMed] [Google Scholar]
- 17.Valenstein E, Bowers D, Verfaellie M, et al. Retrosplenial amnesia. Brain 1987;110:1631–1646 [DOI] [PubMed] [Google Scholar]
- 18.von Cramon DY, Hebel N, Schuri U. A contribution to the anatomical basis of thalamic amnesia. Brain 1985;108:993–1008 [DOI] [PubMed] [Google Scholar]
- 19.Graff-Radford NR, Tranel D, Van Hoesen GW, Brandt JP. Diencephalic amnesia. Brain 1990;113:1–25 [DOI] [PubMed] [Google Scholar]