Abstract
Summary: We present the MR imaging findings of a girl with horizontal gaze palsy and progressive scoliosis (HGPPS). HGPPS is a rare congenital disorder characterized by absence of conjugate horizontal eye movements and accompanied by progressive scoliosis developing in childhood and adolescence. MR imaging depicted brain-stem hypoplasia with absence of the facial colliculi, presence of a deep midline pontine cleft (split pons sign), and a butterfly configuration of the medulla. These MR imaging features suggest the diagnosis of HGPPS and correlate with the clinical findings. We hypothesize that maldevelopment of dorsomedial brain-stem structures plays a crucial role in the pathogenesis of HGPPS.
Horizontal gaze palsy with progressive scoliosis (HGPPS; On-Line Mendelian Inheritance in Man accession number 607313) is a rare autosomal recessive disorder characterized by congenital absence of conjugate horizontal eye movements, preservation of vertical gaze and convergence, and progressive scoliosis developing in childhood and adolescence. We present the MR imaging findings in a patient with HGPPS and discuss the pathogenesis and possible embryologic substratum of this rare entity. We also propose to shed light on the relationships between HGPPS and other congenital gaze palsy syndromes.
Case Report
A 13-year-old girl with early-onset thoracolumbar scoliosis was admitted to our institution to undergo surgical correction of her spinal deformity. She was born at term to healthy nonconsanguineous parents after an uneventful pregnancy. She was noted to have complete absence of horizontal eye movements since the neonatal period, whereas vertical eye movements and convergence were preserved. She compensated for her deficit by turning her head in the desired direction, thereby obtaining regular binocular vision. Neurologic examination was otherwise normal. Thoracolumbar scoliosis was noted at age 18 months by visual inspection; corset treatment was attempted from age 2 to 10 years but failed to halt progression. CT scanning of the brain was performed at an outside institution at age 3 years and was reported to be normal. The girl underwent MR imaging of the entire neuraxis at our institution before spinal surgery (Fig 1). Imaging findings were normal except for double-curve thoracolumbar scoliosis. Brain MR imaging revealed a hypoplastic pons in which the posterior two-thirds were split into two halves by a midsagittal cleft extending ventrally from the fourth ventricular floor, generating a split pons sign on axial images. The facial colliculi were absent, and the fourth ventricular floor was tent shaped. The medulla was also hypoplastic and showed a butterfly configuration. The inferior olivary nuclei were prominent with respect to the pyramids, and the prominence of the gracile and cuneate nuclei on the posterior aspect of the medulla was absent.
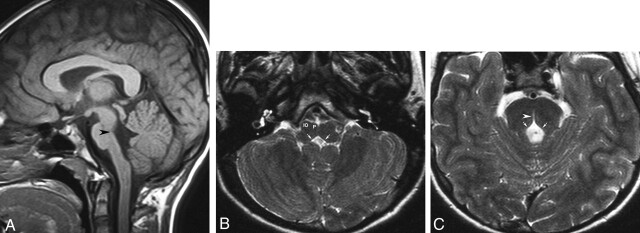
Fig 1.
MR images obtained in a 13-year-old girl with early-onset thoracolumbar scoliosis.
A, Sagittal T1-weighted image (500/12 [TR/TE]) of the brain shows depression of the floor of the fourth ventricle (arrowhead). The pons and medulla oblongata have a reduced volume.
B, Axial T2-weighted image (4500/120) at the level of the medulla oblongata shows rectangular configuration of the medulla. The floor of the fourth ventricle is tent shaped (arrows), with missing prominence of the cuneate and gracile nuclei. The inferior olivary nuclei (IO) are prominent with respect to the pyramids (P).
C, Axial T2-weighted image (4500/120) at the level of the pons shows absence of the facial colliculi, with tent-shaped configuration of the floor of the fourth ventricle (arrows). A deep midsagittal cleft extends ventrally from the fourth ventricular floor, producing the split pons sign (arrowhead).
Discussion
The first descriptions of associated horizontal gaze palsy and scoliosis date back to 30 years. In 1974, Crisfield (1) observed four Chinese siblings with severe scoliosis and progressive external ophthalmoplegia, and Dretakis and Kondoyannis (2) reported five other cases from two nonconsanguineous families. In 1975, Sharpe et al (3) considered the association of paralysis of horizontal gaze, pendular nystagmus, and progressive scoliosis to constitute a distinctive heredofamiliar syndrome. In reviewing the literature more than 20 years later, Thomsen et al (4) found 11 articles reporting 39 patients with the combination of progressive scoliosis and familial congenital gaze palsy. In 2002, Jen et al (5) described six patients from two nonconsanguineous families and mapped the disease locus to a 30-cM interval on chromosome 11q23–25; two additional patients were reported by Pieh et al (6).
To our knowledge, Pieh et al (6) were the only authors to report on MR imaging findings of HGPPS. In that study, two brothers were shown to have an image of brain-stem hypoplasia that was similar, although not completely identical, to what we are reporting here. In both cases, there was reduction in volume of both the pons and medulla. Absence of the facial colliculi and a rectangular shape of the medulla oblongata were the most prominent MR features, although the split pons sign was not observed. We believe that close inspection of the morphology of the brain stem in patients with HGPPS can suggest specific abnormalities of nuclei and tracts that can, in turn, shed light on the pathogenesis of this syndrome.
Smooth horizontal gaze requires that the lateral rectus muscle of one eye (innervated by the abducens nerve) and the medial rectus muscle of the other eye (innervated by the oculomotor nerve) work together. This coordinated activity is controlled by the abducens nucleus. This nucleus contains two populations of neurons: one directly innervating the ipsilateral lateral rectus muscle and the other consisting of internuclear neurons that project through the medial longitudinal fasciculus (MLF) to the contralateral oculomotor nucleus, in which motor neurons innervate the medial rectus of the other eye (7). The abducens nuclei are located in the lower part of the pontine tegmentum at the level of the fourth ventricular floor. They are surrounded by the axons of the motor nuclei of the facial nerve, curving around the abducens nuclei to form the internal genu of the facial nerve. Together, the abducens nuclei and facial nerve roots form the facial colliculi: paired prominences of the fourth ventricular floor that are clearly depicted on axial MR images in healthy individuals. Absence of such prominence in our patient suggests selective agenesis of the abducens nuclei, thereby explaining congenital horizontal gaze palsy. Instead, normal facial nerve function in patients with HGPPS suggests the roots of the facial nerve are normally developed. Remarkably, the conformation of the pons in our patient closely resembles that of early gestational metencephalon, from which the pons is developed. Between gestational weeks 5 and 8, the developing fourth ventricle (Fig 2) shows a ventral furrow that deeply indents the posterior aspect of the metencephalon (8). This furrow will progressively disappear as dorsomedial nuclei and tracts develop. Abnormal development of the abducens nuclei and MLF could result into persistence of an abnormally deep ventral fourth ventricular furrow, thus producing the split pons sign.
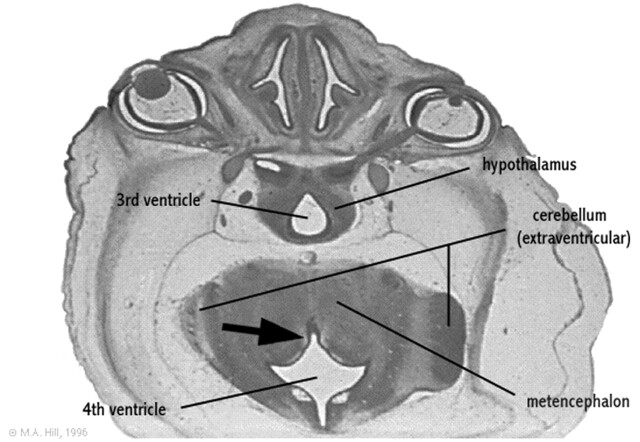
Fig 2.
Rendering of the embryologic development of the fourth ventricle.
Axial section of a 27-mm human embryo (approximately day 56 and Carnegie stage 22) shows ventral fourth ventricular furrow (arrow) deeply indenting the posterior aspect of the developing metencephalon.
Modified from M. Hill, UNSW Embryology, version 3.0 (8), with permission.
The pathogenesis of so-called idiopathic scoliosis remains a subject of debate. It has been suggested that a primary neurologic dysfunction at some level of the central nervous system can be one of the underlying causes (9). Such dysfunction could involve the proprioceptive inputs, mediated by the posterior column pathways of spinal cord and medial lemniscus, the postural equilibrium and labyrinthine function mediated by the vestibular nuclei, and the interplay of visual and vestibular reflexes mediated by the superior colliculus and MLF. Significantly, experimental kyphoscoliosis has been induced in rats by selective brain-stem damage involving the gracile nucleus, the lateral vestibular nucleus, and the superior colliculus (10). Analysis of MR images obtained both in our patient and in those reported in the literature can only allow speculations about the pathogenesis of scoliosis in HGPPS; however, absence of the posterior prominence of the gracilis and cuneate nuclei could reflect hypoplasia of these posterior column nuclei. In addition, hypoplasia of the medial lemniscus, which is located posterior to the pyramids in the normal brain, could explain why the pyramids are not prominent with respect to the inferior olivary nuclei. These observations suggest a speculation that proprioceptive input pathways are not well developed in patients with HGPPS. Moreover, the split pons sign could reflect maldevelopment of dorsomedial pontine structures, including in addition to the abducens nuclei, the MLF. Significantly, the MLF comprises fibers descending to the spinal cord from various brain-stem nuclei (medial vestibular nucleus, interstitial nucleus of Cajal, superior colliculus, and pontine reticular formation) and is involved in automatic postural, visual, and head coordinating activities and proximal trunk movements (7).
HGPPS is one of several genetic disorders of eye and lid control that are believed to result from cranial nuclear maldevelopment (11). Among these entities, the most closely related to HGPPS are Duane retraction syndrome and Möbius syndrome. In fact, abnormal development of the abducens nucleus plays a crucial role in the pathogenesis of both these entities, as well as of HGPPS. Duane retraction syndrome is characterized by absent abduction, insufficient adduction, globe retraction, and palpebral narrowing during fixation (12). It is caused by selective absence of direct abducens nerve motoneurons with preservation of internuclear neurons. Thus, affected patients lack abduction, but the contralateral eye can adduct on attempted lateral gaze (11). Möbius syndrome is characterized by complete or partial facial diplegia associated with horizontal gaze palsy and, frequently, dysfunction of other cranial nerves; it is caused by agenesis or destruction of both the abducens and facial nuclei, possibly accompanied by abnormalities of other cranial nerve nuclei (13). MR imaging findings in both Duane retraction syndrome and Möbius syndrome have only rarely been reported. In one study (12), the abducens nerve on the affected side could not be visualized by use of high-resolution MR imaging in 54% of eyes affected by Duane retraction syndrome; however, the morphology of the facial colliculi was not commented upon. In a study of three patients with Möbius syndrome, absence of the facial colliculus was identified (13). In Möbius syndrome, this feature suggests hypoplasia of both the abducens and facial nuclei, which differs from HGPPS; absence of the facial colliculus results from agenesis of the abducens nucleus alone. It is possible that the greater degree of brain-stem hypoplasia seen in patients with HGPPS, characterized by involvement of both the pons and medulla, reflects abnormality of additional cell groups that are required for maintenance of axial posture, thus explaining the association of scoliosis (11). Further studies with larger series are needed to define the role of MR imaging in the differential diagnosis of congenital eye movement disorders. We suggest that diffusion tensor imaging could play an important role in the identification of abnormalities involving specific brain-stem tracts and nuclei.
Our results support the theory that maldevelopment of dorsomedial brain-stem structures plays a crucial role in the pathogenesis of HGPPS. Horizontal gaze palsy is explained by agenesis of abducens nuclei, whereas scoliosis could be due to chronic muscle tone abnormalities resulting from primary brain-stem disease involving posterior column structures and associative bundles. MR imaging reveals brain-stem hypoplasia and can suggest abnormalities of specific brain-stem structures in these patients.
References
- 1.Crisfield RJ. Scoliosis with progressive external ophthalmoplegia in four siblings. J Bone Joint Surg 1974;56B:484–489 [PubMed] [Google Scholar]
- 2.Dretakis EK, Kondoyannis PN. Congenital scoliosis associated with encephalopathy in five children of two families. J Bone Joint Surg 1974;56A:1747–1750 [PubMed] [Google Scholar]
- 3.Sharpe JA, Silversides JL, Blair RD. Familial paralysis of horizontal gaze: associated with pendular nystagmus, progressive scoliosis, and facial contraction with myokymia. Neurology 1975;25:1035–1040 [DOI] [PubMed] [Google Scholar]
- 4.Thomsen M, Steffen H, Sabo D, Niethard FU. Juvenile progressive scoliosis and congenital horizontal gaze palsy. J Pediatr Orthop B 1996;5:185–189 [PubMed] [Google Scholar]
- 5.Jen J, Coulin CJ, Bosley TM, et al. Familial horizontal gaze palsy with progressive scoliosis maps to chromosome 11q23–25. Neurology 2002;59:432–435 [DOI] [PubMed] [Google Scholar]
- 6.Pieh C, Lengyel D, Neff A, et al. Brainstem hypoplasia in familial horizontal gaze palsy and scoliosis. Neurology 2002;59:462–463 [DOI] [PubMed] [Google Scholar]
- 7.Sundsten JW, Mulligan KA. Neuroanatomy interactive syllabus. University of Washington,1998. . Available online at http://www9.biostr.washington.edu/cgi-bin/DA/imageform (accessed July 27, 2003)
- 8.Hill M. UNSW embryology, version 3.0. University of New South Wales,2003. . Available online at http://anatomy.med.unsw.edu.au/cbl/embryo/Embryo.htm (accessed July 27, 2003)
- 9.Lowe TG, Edgar M, Margulies JY, et al. Etiology of idiopathic scoliosis: current trends in research. J Bone Joint Surg Am 2000;82-A:1157–1168 [DOI] [PubMed] [Google Scholar]
- 10.Barrios C, Arrotegui JI. Experimental kyphoscoliosis induced in rats by selective brain stem damage. Int Orthop 1992;16:146–151 [DOI] [PubMed] [Google Scholar]
- 11.Engle EC, Leigh RJ. Genes, brainstem development, and eye movements. Neurology 2002;59:304–305 [DOI] [PubMed] [Google Scholar]
- 12.Ozkurt H, Basak M, Oral Y, Ozkurt Y. Magnetic resonance imaging in Duane’s retraction syndrome. J Pediatr Ophthalmol Strabismus 2003;40:19–22 [DOI] [PubMed] [Google Scholar]
- 13.Pedraza S, Gamez J, Rovira A, et al. MRI findings in Möbius syndrome: correlation with clinical features. Neurology 2000;55:1058–1060 [DOI] [PubMed] [Google Scholar]