Abstract
Summary: We describe a case of midcervical angiosarcoma causing compression of the cervical spinal cord, producing rapidly progressive neurologic deficits. The tumor had recurred despite previous resection and was refractory to radiation and chemotherapy. Shrinkage of the tumor by percutaneous embolization was not considered feasible. A single direct percutaneous intratumoral injection of 15 U of bleomycin produced sufficient tumor shrinkage to relieve the pressure on the spinal cord and thereby reverse some of the neurologic deficits and give adequate palliation against recurrence of this problem for the remainder of the patient’s life. Direct percutaneous intratumoral injection of bleomycin may thus be considered for palliation when other treatment methods have failed to elicit a suitable clinical response.
The direct intralesional administration of chemotherapeutic agents into tumors is described and has been used for the treatment of cerebral tumors such as craniophanyngiomas and glioblastoma multiforme (1–3). Celikoglu et al (4) relieved malignant branchial obstruction in 81 of 93 patients by injecting a mixture of chemotherapeutic agents directly into endobronchial tumors with the aid of a flexible fiber-optic bronchoscope. We describe our recent experience in a single case in which direct intratumoral injection of bleomycin was used to reverse progressive neurologic deficits resulting from cervical spinal cord compression due to angiosarcoma.
Case Report
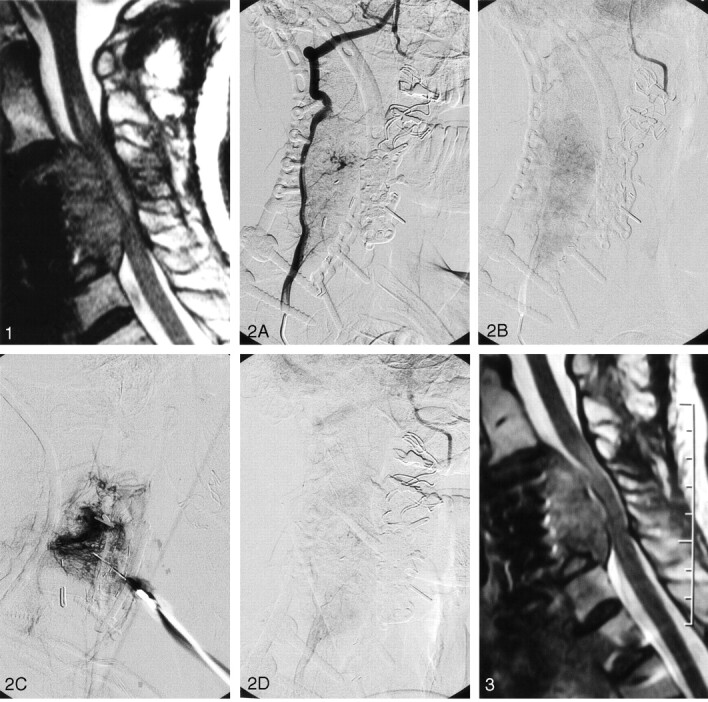
A 36-year-old male patient presented with a malignant tumor of the midcervical spine, described histologically as an angiosarcoma. Previous corpectomies of the C3 and C4 vertebral bodies had been performed with secondary anterior and posterior cervical fusions to maintain structural stability of the neck. Despite previous surgery and two previous sessions of radiation therapy, the tumor had continued to enlarge. The patient presented with threatening quadriplegia with rapidly progressive loss of function of the right arm (already partly monoparetic probably because of brachial plexus involvement) and right leg, the patient being unable to stand or elevate the leg against gravity. He also complained of intermittent local neck pain and spasm. MR imaging showed extension of the tumor into the anterior cervical canal with compression of the cervical cord and cord edema (Fig 1). He was placed on high-dose oral dexamethosone therapy, but had shown little clinical improvement from it. He was then referred for possible embolization of the tumor to stay the progression of the neurologic deficits. The left vertebral artery had already been sacrificed during previous surgical procedures. Right vertebral and ascending cervical arteriography showed narrowing of the right vertebral artery at the level of the tumor, with only a mild tumor blush seen with supply to the tumor by small segmental branches of the vertebral artery itself (Fig 2A and B). Very little visible supply to the tumor was also noted via the ascending and deep cervical arteries.
Fig 1.
Sagittal T2-weighted MR image shows the tumor compressing the cervical cord, with associated cord edema.
Fig 2.

Anteriograms obtained before, during and after the bleomycin injection. A, Selective vertebral digital subtraction arteriogram, right anterior oblique projection, shows narrowing due to tumor encasement and the related arterial supply to the tumor.
B, Same run, late capillary phase, shows the degree of tumor blush.
C, Direct percutaneous injection of contrast medium is given to assess the volume of bleomycin to be injected and to estimate the approximate pressure by which it is to be injected.
D, Following injection of the bleomycin, a check vertebral arteriogram shows an area of reduced opacification corresponding to that into which the bleomycin was injected.
It was decided that embolization would not only be difficult to perform, but would also probably not achieve much in light of the relatively avascular nature of the tumor and that the risk-benefit ratio would be unacceptably high. Direct percutaneous injection of the tumor was then performed by using bleomycin (Blenoxane, Bristol-Meyers Squibb). Fifteen units (1 vial) of bleomycin were dissolved in 15 mL of sterile water. Iodinated contrast medium (Visipaque, Nycomed, Bedfordview, South Africa) was initially injected into the tumor under fluoroscopic guidance to assess the volume of bleomycin to be injected and the approximate pressure at which contrast would reflux into the vertebral artery (Fig 2C). The bleomycin mixture was then injected slowly and with a subsystolic pressure directly into the posteriomedial part of the tumor, where it was seen to be encroaching upon the anterior canal and cervical cord. Check vertebral arteriography was then performed, which showed a reduced to absent contrast material blush on the capillary-phase images in the areas in which the bleomycin had been injected (Fig 2D). Arteriography and intralesional bleomycin injection (IBI) were performed under general anesthesia. After the anesthetic was administered, the patient complained of local pain and neck spasm induced by a coughing bout during recovery from the anesthetic that resolved with minor analgesic and nonsteroidal anti-inflammatory drugs.
During the following 4 weeks, he made a good clinical recovery, regaining significant use of his right leg to the point that he could stand unaided. There had only been slight improvement in the strength and function of the right arm. No further local neck pain was felt. A repeat MR image was obtained 4 weeks after the first injection. This showed a reduction of the volume of the tumor in its posterior aspect at the site of the bleomycin injection with less visible compression on and displacement of the cervical cord (Fig 3). Inhomogeneous signal intensity was also noted within the tumor on the T2-weighted images, which suggested areas of tumor necrosis.
Fig 3.

Sagittal T2-weighted MR image obtained 4 weeks after the initial bleomycin injection shows the degree of shrinkage of the tumor with less compression of the cord.
A second IBI procedure was performed 4 weeks after the initial one. Again, a total of 15 U of bleomycin was injected into the tumor, but more so into the central and lateral aspects of the tumor. No postprocedural pain or neurologic deterioration was noted. The oral steroid dosage was then progressively reduced from this point. Another follow-up MR image was obtained 3 weeks later, which showed no further visible reduction of the size of the tumor. A third IBI procedure was then performed. At this stage, however, the patient had developed acute-onset, severe pain in the left hip region and right anterior chest wall. An MR image of the pelvis and hip region showed a large metastatic deposit in the left femoral head and neck but with no associated pathologic fracture. Numerous other metastatic deposits were also seen in the rest of the bony pelvis, as well as in the right first rib and the sternum. No further IBI treatment was given, and the patient died of metastatic disease 8 weeks later. During this period, he developed no further neurologic deterioration or local pain related to the neck lesion.
Discussion
Bleomycin is an antibiotic derivative with cytostatic properties that was first approved by the United States Food and Drug Administration in 1975 for therapy against squamous cell carcinomas, testicular cancers, and malignant lymphomas (5). Bleomycin is also approved for intrapleural administration for the treatment of malignant pleural effusions. Although bleomycin has previously been administered directly into tumors, generally into the cystic aspects of certain cerebral tumors (1–3), it is not currently approved for direct intratumoral injection. Its antimitotic activity results from producing DNA strand breads mainly in M and G2 phase cells, possibly mediated by the production of intracellular free radicals (5, 6). Pingyangmycin, a similar compound available in Asia and the active component of which is bleomycin A5, has been shown to kill cells by necrosis (reproductive cell death) at low concentrations and by apoptosis (programmed cell death) at higher concentrations (7). The first description of the antiangiogenic properties of bleomycin was by Oikawa et al (8) in 1990. These were later again confirmed experimentally by Licun and Gongjia (9). IBI has been used with very good results for the treatment of lymphatic malformations and hemangiomas (10–12). On the basis of our own recent experience with the treatment of low-flow venous and lymphatic malformations and hemangiomas with IBI, we have also become familiar with the antiangiogenic properties of bleomycin (13, 14). Thus, we chose to use bleomycin as the agent for direct intratumoral administration in our case on the basis of its known antimitotic and antiangiogenic properties.
Before arteriography, which was to be performed under general anesthesia, the possibility of transarterial embolization of the tumor was discussed with the patient, as was the alternative untested option of direct intratumoral injection of bleomycin if embolization was not viable. Our rationale for attempting this treatment was explained together with the possible outcomes and complications. In view of the rapidly deteriorating clinical situation and lack of any viable alternative treatment method, our patient agreed to undergo the bleomycin injections if transarterial embolization was not performed. The bleomycin would then be given as per the IBI protocol of the Pretoria Vascular Malformation Group (13, 14). The dose given (15 U per session) was the same average maximal dose given to our adult patients for the treatment of low-flow vascular malformations.
We can offer no detailed explanation as to the exact method of action of the bleomycin in our case, but have merely observed the clinical response. This response was probably due to a combination of the antimitotic, apoptotic and antiangiogenic properties, all coupled with a high local concentration of the drug in the tumor due to its direct delivery. We can also not explain the lesser response of the tumor to the second IBI. The goal of treatment being to halt and even reverse the progression of the neurologic deficits had been achieved after the first IBI. This produced excellent palliation of symptoms for the remainder of our patient’s life.
Conclusion
Direct percutaneous intralesional injection of chemotherapeutic agents such as bleomycin can be considered as an alternative method of treatment in some tumors that have failed to respond to more conventional methods. Bleomycin has both antimitotic and antiangiogenic properties that make it a useful agent to consider for direct intralesional delivery.
References
- 1.Alen JF, Boto GR, Lagares A, et al. Intratumoural bleomycin as a treatment for recurrent cystic craniopharyngioma: case report and review of the literature. Neurocirugia 2002;13:479–485 [DOI] [PubMed] [Google Scholar]
- 2.Jiang R, Liu Z, Zhu C. Preliminary exploration of the clinical affect of bleomycin on craniopharyngiomas. Sterotact Funct Neurosurg 2002;78:84–94 [DOI] [PubMed] [Google Scholar]
- 3.Patchell RA, Regine WF, Ashton P, et al. A phase 1 trial of continuously infused intratumoural bleomycin for the treatment of recurrent glioblastoma multiforme. J Neurooncol 2002;60:37–42 [DOI] [PubMed] [Google Scholar]
- 4.Celikoglu SI, Karayel T, Demirci S, et al. Direct injection of anti-cancer drugs into endobronchial tumours for palliation of major airway obstruction. Postgrad Med J 1997;73:159–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett JM, Reich SD. Bleomycin. Ann Intern Med 1979;90:945–948 [DOI] [PubMed] [Google Scholar]
- 6.Das UN. A radical approach to cancer. Med Sci Monit 2002;8:RA79–RA92 [PubMed] [Google Scholar]
- 7.Tai KW, Chou MY, Hu CC, et al. Induction of aptoptosis in KB cells by pingyangmycin. Oral Oncol 2000;36:242–247 [DOI] [PubMed] [Google Scholar]
- 8.Oikawa T, Hirotani K, Ogasawara H, et al. Inhibition of angiogenesis by bleomycin and its copper complex. Chem Pharm Bull (Tokyo) 1990;36:1790–1792 [DOI] [PubMed] [Google Scholar]
- 9.Licun W, Gongjia S. Treatment of hemangioma with angiogenesis inhibitor pingyangmycin. Indian Pediatr 2000;37:636–639 [PubMed] [Google Scholar]
- 10.Sanlialp I, Karnak I, Tanyel FC, et al. Sclerotherapy for lymphagioma in children. Int J Pediatr Otorhinolaryngol 2003;67:795–800 [DOI] [PubMed] [Google Scholar]
- 11.Kullendorf CM. Efficacy of bleomycin treatment for symptomatic hemangiomas in children. Pediatr Surg Int 1997;12:526–528 [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Gao Q, Fu F, et al. Treatment of hemangioma in oral and maxillofacial region with pingyangmycin injection [abstract]. Hua Xi Kou Qiang Yi Xue Za Zhi 2000;18:317–319 [PubMed] [Google Scholar]
- 13.Muir T, Kirsten M, Fourie P, et al. Intralesional bleomycin injection (IBI) treatment for hemangiomas and congenital vascular malformations. Pediatr Surg Int 2004;19:766–773 [DOI] [PubMed] [Google Scholar]
- 14.Fourie PA, Ionesco GO, Muir T, Coetzee PF. Results of 95 patients with hemangiomas and vascular malformations treated with intralesional bleomycin [abstract]. Cardiovasc Intervent Radiol 2002;25(Suppl 2):S163 [Google Scholar]


