Abstract
BACKGROUND AND PURPOSE: If tumor volumes are to be used for evaluating responses to treatment and long-term outcomes of patients with primary pharyngeal carcinomas, the reproducibility of these measurements must be established. We determined the intraobserver variability of MR imaging–based volume measurements of these cancers and their regional metastases.
METHODS: We used an interactive computer program (IDL) that enables the extraction of tumor volumes from 3D MR data to obtain 202 volume measurements in 17 patients with pharyngeal carcinoma (two to five time points each). The primary cancer and largest nodal mass were manually outlined on every T2-weighted image of each MR study. The same neuroradiologist reanalyzed this MR dataset 2–41 weeks later. Measurement error and percentage measurement error (intraobserver variability) were determined. Differences in intraobserver variability between primary lesions and nodes, as well as between stages of treatment were tested with a Wilcoxon rank sum test.
RESULTS: The mean and median percentage measurement errors, respectively, were 13% and 12% (range, 0–53%; 95% CI: 10%, 16%) for primary tumors and 9% and 7% (range, 0–37%; 95% CI: 7%, 12%) for nodal metastases. The difference in the percentage measurement error between primary lesions and cervical nodes approached statistical significance (P = .07). Differences in the variation of volume measurements based on the stage of therapy were significant (P = .01).
CONCLUSION: Our results suggest that MR imaging–based tumor volumes are reliably reproducible. Such measurements may be important in predicting patient outcome, determining appropriate therapy, and conducting patient follow-up.
According to the Cancer FactBook of the National Cancer Institute, squamous cell carcinoma of the head and neck was responsible for almost 12,000 deaths in 2001 (1). Patients with head and neck cancer have an estimated overall 5-year survival rate of 40–60% (1). Therapeutic options usually include surgery or irradiation or both. However, concurrent chemotherapy and radiation therapy is increasingly used to treat patients with this cancer. Specific management depends on the stage of the tumor, the patient’s preference, the patient’s general underlying medical condition, as well as the institution where the patient will be treated. To tailor therapy and maximize the potential impact of treatment options, it is necessary to have prognostic factors and a reliable means by which to accurately stage the disease before treatment. Also needed is a quantitative measure to evaluate the response to therapy.
The literature suggests that pretreatment tumor volumes may be reliable predictors of both local control and overall survival in patients with head and neck cancer treated with irradiation (2–12). In general, larger lesions have poorer control rates, although the critical volumes necessary for local control are variable and depend on the location of the primary tumor (2–8). In addition, investigators have suggested that tumor volumes may also be useful in predicting local control in laryngeal carcinoma treated with surgery (13, 14).
Tumor volumes may serve as not only prognostic variables but also quantitative measures of the response to therapy. Currently, both clinical assessment and cross-sectional imaging are used to monitor responses to treatment. Clinical determination of tumor size alone is more subjective and requires sophisticated equipment combined with a high level of expertise, which may not be available away from specialized medical centers (8, 15–17). While mucosal lesions are well visualized endoscopically, clinical examination does not permit adequate assessment of many head and neck cancers because of the submucosal extension of disease.
CT and MR imaging are currently the most accurate and reproducible methods available to measure the response of cancers to therapy (17). Tumor size is usually determined by measurements made in one or two dimensions (17, 18). More recently, tumor volumes determined by using CT measurements have been used to assess tumor burden. Although CT is effective in identifying submucosal disease, inaccuracies in defining tumor boundaries are common because edema and tumor can be difficult to distinguish and because the attenuation of tumor and that of adjacent normal structures (eg, muscle) frequently overlap (19–26). MR imaging is reportedly more accurate than CT in assessing extension of the primary tumor (15, 19–21, 27). Like CT, MR imaging may not be reliable in differentiating edema from tumor (19, 28); however, it is more sensitive in distinguishing abnormal tissue (edema and neoplasm) from normal adjacent structures (15, 20, 21, 27).
If tumor volumes are to be used in clinical practice and in prospective studies as a standard for determining responses to treatment and, potentially, long-term outcomes, it is necessary to establish the reproducibility of these measurements (29, 30). The purpose of this study was to examine the intraobserver variability of MR imaging–based tumor volumes in squamous cell cancers of the pharynx. We studied volume measurements of primary tumors and cervical nodal metastases before and during treatment (irradiation or chemotherapy or both).
Methods
We studied serial MR images of 17 consecutive male patients aged 34–72 years (mean, 54 years), with newly diagnosed, pathologically proved, squamous cell carcinoma of the pharynx (Table 1). Sixteen patients had cervical nodal metastases (Table 2). The patients were taken from a cohort enrolled in a prospective National Institutes of Healthy study analyzing MR spectroscopy to predict the responses of head and neck cancer to therapy. All but two patients underwent MR study before treatment followed by one to four additional MR studies during treatment.
TABLE 1:
Characteristics of primary cancers
| Patient (No.) | Tumor Location | Stage | MR Imaging Time Points | Percentage Measurement Error, % |
|---|---|---|---|---|
| 1 | Base of tongue | T4 | 2 | 23*, 19 |
| 2 | Pyriform sinus | T4 | 3 | 18, 20, 16 |
| 3 | Base of tongue | T3 | 5 | 5, 7, 12, 17, 2 |
| 4 | Base of tongue | T4 | 1 | 0 |
| 5 | Tonsil | T4 | 3 | 1, 2, 10 |
| 6 | Tonsil | T3 | 4 | 1, 3, 22†, 29† |
| 7 | Base of tongue | T3 | 0‡ | None |
| 8 | Tonsil | T4 | 3 | 6, 15, 53† |
| 9 | Base of tongue | T2 | 4 | 13, 8, 37*, 11 |
| 10 | Base of tongue | T3 | 2 | 16, 11 |
| 11 | Base of tongue | T2 | 3 | 1, 3, 12 |
| 12 | Base of tongue | T2 | 3§ | 29*, 22*, 2 |
| 13 | Base of tongue | T3 | 3 | 4, 8, 19 |
| 14 | Base of tongue | T4 | 3 | 9, 3, 22† |
| 15 | Pyriform sinus | T4 | 3 | 7, 2, 23† |
| 16 | Tonsil | T2 | 3 | 0, 13, 26† |
| 17 | Base of tongue | T2 | 3 | 4, 4, 2 |
Percentage measurement error was greater than 20% because of difficulty in differentiating the neoplasm from adjacent lymphoid tissue at the base of the tongue.
Percentage measurement error was greater than 20% because of a small absolute differences in the volume measurements of small lesions.
The primary tumor was too large to measure.
T1-weighted images were used because of artifact on T2-weighted images.
TABLE 2:
Characteristics of regional nodal metastases
| Patient (No.) | Stage | Nodal Level | MR Imaging Time Points | Percentage Measurement Error, % |
|---|---|---|---|---|
| 1 | N0 | None | 0* | None |
| 2 | N2c | L II/III | 4 | 14, 13, 11, 11 |
| 3 | N2b | R II | 5 | 2, 1, 3, 9, 31† |
| 4 | N2c | R II/III | 3 | 2, 7, 3 |
| 5 | N2c | R II/III | 3 | 1, 2, 10 |
| 6 | N2b | L II-IV | 4 | 1, 1, 3, 8 |
| 7 | N2c | R II/III | 3 | 14, 26‡, 4 |
| 8 | N2a | R II | 3 | 5, 1, 4 |
| 9 | N2b | R II | 4 | 4, 12, 0, 11 |
| 10 | N2b | R II/III | 2 | 3, 30† |
| 11 | N2a | L II | 3 | 4, 1, 1 |
| 12 | N3 | R II | 3 | 17, 16, 27* |
| 13 | N2b | R II | 3 | 3, 17, 17 |
| 14 | N2c | L II | 4 | 4, 16, 13, 19 |
| 15 | N3 | R I-V | 3 | 3, 7, 20 |
| 16 | N2b | R II | 3 | 4, 9, 37† |
| 17 | N2c | L II/III | 3 | 9, 6, 4 |
No nodal metastases.
Percentage measurement error was greater than 20% because of small absolute differences in the volume measurements of small lesions.
Percentage measurement error was greater than 20% because of inaccuracies in the volume measurements due to patient motion.
MR images were obtained on the same 1.5-T system (Signa; GE Medical Systems, Milwaukee, WI). Imaging consisted of conventional spin-echo imaging with an anteroposterior neck coil. Acquisition consisted of 5-mm-thick interleaved sagittal T1-weighted images (TR/TE/NEX, 600/10/2), axial T1-weighted images (600/10/2), and axial fat-suppressed fast spin-echo T2-weighted images (4000/100/2). Other imaging parameters included a 24-cm field of view, a 256 × 128 matrix for sagittal and axial T1-weighted images, and a 256 × 192 matrix for axial T2-weighted images.
MR images were transferred to an interactive computer program (IDL) developed at our institution that enables the extraction of tumor volumes from the 3D MR data (31). Calculations with this program were previously described (31) and were used to estimate the volume of spherical phantoms to within 1% of their true volume. A neuroradiologist (L.A.L.) reviewed the images obtained with all sequences and then used the sequential, axial T2-weighted images to outline the primary tumor by using a mouse-controlled cursor (Fig 1). The entire area of abnormal T2 signal intensity in the region of interest was included in the volumetric analysis. At a separate reading, the same method was used to assess the largest nodal mass (Fig 2). The computer program calculated the volume by only counting the voxels included within the outlined regions, with a further correction algorithm used for partial volume effects (31). The voxel volumes generated (in the x, y, and z dimensions) were then converted to physical units in cubic centimeters by using the following equation: (xFOV/xRES) × (yFOV/yRES) × thickness = (24 cm/256) × (24 cm/256) × 0.5 cm = 4.3945 × 10−3 cm3 = one pixel, where FOV is the field of view and RES is the resolution.
Fig 1.
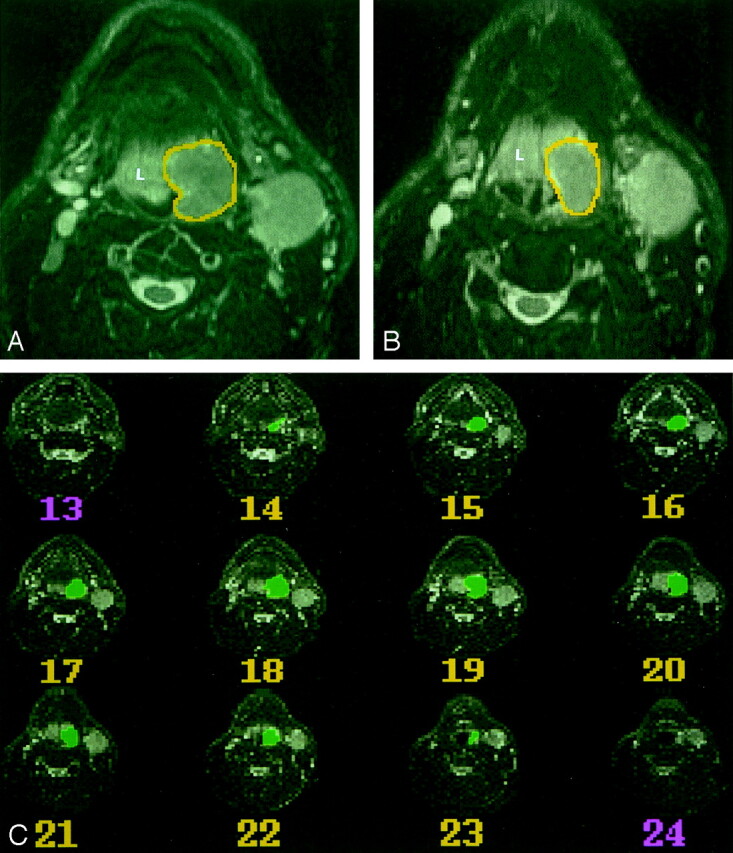
MR imaging–based volumetric analysis of a carcinoma on the left side of the base of the tongue in a 54-year-old man.
A and B, Two sequential axial T2-weighted MR images (4000/100/2) were used to outline the primary tumor on each sequential axial image by using a mouse-controlled cursor. In this case, the neoplasm (outlined) is hypointense relative to the lymphoid tissue (L) of the lingual tonsil.
C, Serial axial T2-weighted MR images (4000/100/2) show all images where a primary tumor is present, and the outlines of the tumor on each section. A computer program calculated the volume by counting voxels included within the outlined regions.
Fig 2.
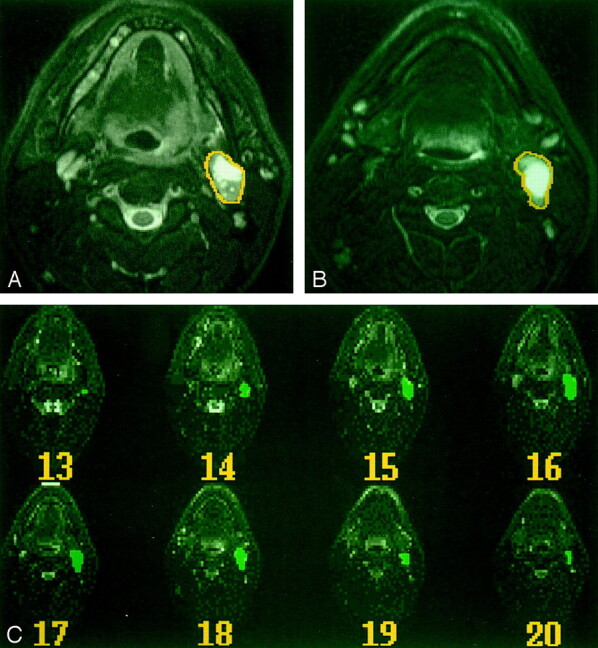
MR imaging–based volumetric analysis of a regional nodal metastasis in a patient with a primary cancer at the base of the tongue.
A and B, Two sequential axial T2-weighted images show how the nodal mass was outlined within a mouse-controlled cursor.
C, Serial axial T2-weighted MR images (4000/100/2) show all images on which nodal metastases are outlined and the outlines on each image.
Images from each MR study were reviewed twice by the same neuroradiologist subspecialized in head and neck imaging who was responsible for all such studies at our institution. To minimize potential learning effects, the primary tumors and largest nodal mass in each patient were measured at two independent readings separated by 2–41 weeks (mean, 20 weeks). During a reading for a single patient, images from every MR study (before and during therapy) were reviewed so that measurements were consistent. (All potential errors were included at all time points.) The results of the first review were not available at the second reading. A total of 202 volume measurements were obtained for 16 primary tumors and 16 nodal masses; each was measured at two to five time points and analyzed at two separate times (Tables 1 and 2).
For statistical analysis, measurement error was calculated as the absolute value of the difference between the measured volumes of the same lesions at the two readings. The percentage measurement error (intraobserver variability) was determined by dividing the measurement error by the average of the two measurements for each lesion (Tables 1 and 2). The measurement error is reported as a percentage, rather than an absolute difference, to incorporate our results into lesions of all sizes. The mean, median, standard deviation, and 95% confidence interval (CI) were calculated for the primary cancers and the nodal masses. Differences in measurement error between primary lesions versus nodes and between different stages of treatment were tested with a Wilcoxon rank sum test. For primary lesions, the effect of lesion subsite in the pharynx on measurement error was tested with a Kruskal-Wallis rank sum test. Nonparametric tests were used because the data were not normally distributed. For all statistical tests, significance was defined as P < .05.
Results
The measured volume of the primary cancers were 0.6–65.4 cm3 (average of the two readings) with a mean and median of 14 and 6.9 cm3, respectively. The measured volume of the nodal metastases was 0.24–127.4 cm3, with a mean and median of 12.9 and 6.2 cm3, respectively.
The percentage measurement error for primary tumors was 0–53%, with a mean of 13% and a median of 12% (95% CI: 10%, 16%). The percentage measurement error of 53% corresponded to an absolute measurement error of 3.02 cm3. When this measurement was categorized as an outlier, the next largest percentage measurement error for primary cancers was 37%. The percentage measurement error for the cervical nodes was 0–37%, with a mean of 9% and a median of 7% (95% CI: 7%, 12%) (Table 3).
TABLE 3:
Percentage measurement error for primary pharyngeal cancer and regional nodal metastases
| Percent Measurement Error | Primary Tumor | Nodal Mass |
|---|---|---|
| Mean, % | 13 | 9 |
| Standard deviation, % | 11 | 9 |
| Median, % | 12 | 7 |
| 95% CI, % | 10,16 | 7,12 |
| Range, % | 0,53 | 0,37 |
No statistically significant difference was found for the variation in primary tumor volume measurements based on the subsite in the pharynx (base of the tongue, tonsil, and pyriform sinus) (P = .35). The variability of volume measurements based on stage of therapy was statistically significant (P = .01). Pretreatment volume measurements had significantly less variation than measurements made during therapy (mean, 7% vs 13%). The difference in the percentage measurement error between the primary lesions and cervical nodes approached statistical significance (P = .07).
Discussion
Tumor volumes provide quantitative measures that can potentially be used to select treatment for patients with cancer of the head and neck, as well as to predict patient outcomes. Specifically, investigations suggest that pretreatment tumor volumes may help predict what therapy offers the best likelihood of cure for the patient (2–4, 7–14, 23, 32–34). Furthermore, responses reflected by objective changes in tumor volume during the course of treatment may determine if a change in therapy is indicated (17).
Some investigators are using tumor volumes to predict local control and survival rates in patients with head and neck cancer treated with irradiation (2–12, 33). An association between tumor volume and the number of clonogenic cells that must be sterilized by radiation has been reported (9, 11, 35). Currently, CT is the imaging technique predominantly used in the volumetric analysis of primary tumors in cancer patients (2–4, 6, 8, 9, 11–14, 23, 33, 36, 37). Some have suggested threshold volumes in head and neck cancer based on subsite; above these thresholds, local control rates are poor (3, 4, 6, 8, 23). Tumor volumes may also help predict the local control of laryngeal carcinoma treated with surgery (13, 14). The few studies that have used MR imaging–derived tumor volumes have also shown an association between tumor volume and regional control (5, 7).
The role of cervical nodal volumes as a prognostic factor for head and neck cancer treated with radiation therapy has been more variable (8–12, 33). Most studies combined the volumes of the cervical nodes with those of the primary lesion, and the nodal mass was not assessed independently. The volume of cervical nodal metastases may prove to be an independent predictor of local control or patient survival or both (8, 10, 12, 33). This issue is clinically important because many patients with cervical nodes larger than 2–3 cm who undergo neck dissection after radiation therapy (because of uncertainty of control) may be able to avoid unnecessary surgery. To our knowledge, studies of MR imaging–based volume measurements to specifically examine regional metastases have not been performed.
Many methods have been used to calculate tumor volumes from cross-sectional images. Most of these systems involve manually tracing a tumor on hard-copy images, digitizing the outlines, and then transferring the images to a computer where the summation-of-areas technique or a cuboid formula are used to calculate the volume (2–6, 8, 9, 11–14, 33, 37). Volumes generated from CT are generally within 10–20% of the true tumor volumes (3, 4, 6, 13, 23). We used a computer program that generates accurate measurements of volume directly from the 3D MR data (31). Manually outlining the region of interest on the computer allowed us to include every contour of an irregularly shaped mass in the volumetric analysis (Figs 1 and 2).
Imaging-based tumor volumes must be reproducible to define the critical volumes that affect therapeutic decision making and to enable their use as variables in predicting outcomes or in monitoring responses to treatment. The reproducibility is as important as the absolute accuracy of individual tumor volumes in detecting changes over time (36, 38). Few studies have addressed the reproducibility of volumetric measurements in head and neck cancer. Hermans et al (37) found that the pooled coefficient of variation (for all observers and sessions) in CT-based volume measurements of laryngeal tumors was 16.5–114%. Rasch et al (39) found that MR imaging–derived volumes of head and neck tumors had less interobserver variation than the volumes derived from CT (intraobserver variation was not examined). To our knowledge, no studies have expressly examined the reproducibility of volumes for regional metastases.
We specifically examined the intraobserver variability in tumor volume measurements in pharyngeal carcinoma. Interobserver variability was not a focus of this study. Our mean and median percentage measurement errors for primary tumors were 13% and 12%, respectively (95% CI: 10%, 16%) (Table 3). The degree of measurement error is important in determining if changes in tumor volume occur over the course of therapy or if the changes in measured volumes are due to intraobserver variability. At our institution, tumor response is determined both clinically and radiologically by using MR imaging or CT. Patients treated with chemotherapy and irradiation undergo CT or MR studies both during as well as 6–8 weeks after the completion of all therapy. The goal of treatment is a complete response, which is defined as a 100% reduction in the size of the primary lesion (18). In cases in which residual disease are hard to distinguish from treatment-induced changes, short-term follow-up imaging, biopsy, and/or positron emission tomography imaging may be necessary (17). Patients who are initially treated with only chemotherapy undergo CT or MR study after two cycles of chemotherapy. If the primary tumor is reduced by 50% (ie, partial response) (18), irradiation is added to the chemotherapy. When the response is less than 50% (stable or progressive disease) (18), patients go on to salvage surgery or intensive chemotherapy and hyperfractionated irradiation. On the basis of our percentage measurement error (95% CI: 10%, 16%), MR imaging–based tumor volume reductions of greater than 66% can reliably be classified as a partial clinical response at least, and standard irradiation can be added to the chemotherapy regimen. Volume reductions of less than 34% can reliably be classified as no response or progressive disease, and salvage surgery or intensive chemotherapy and hyperfractionated irradiation may be indicated. Volume reductions of 34–66% may be indeterminate, and further clinical or radiologic evaluation may be necessary.
In our study, the measurement error for primary tumors was not significantly affected by subsite in the pharynx. However, our 17 patients had 11 cancers at the base of the tongue but only four tonsillar and two pyriform sinus cancers. Because the base of the tongue often has lymphoid tissue that can be indistinguishable from neoplasm, we speculated that variations in volume measurements at this subsite were greater than those at other locations. In fact, we found that some of the tongue-base lesions that we examined had percentage measurement errors greater than 20% because of the difficulty in differentiating neoplasm from adjacent lymphoid tissue (Table 1). Therefore, a larger sample may reveal the true differences on the basis of subsite.
The mean and median percentage measurement errors for nodal volumes were 9% and 7%, respectively (95% CI: 7%, 12%) (Table 3). The difference in the variability of volume measurements between primary tumors and cervical nodal metastases approached statistical significance (P = .07). Head and neck cancers frequently have poorly defined margins and irregular contours, which can make them difficult to accurately trace. In contrast, nodal metastases are often spherical and better demarcated; therefore, their tracings may be more reliable. At our institution, patients initially treated with irradiation or chemotherapy or both subsequently undergo neck dissection when pretreatment nodes are larger than 3 cm in their maximal dimension. When pretreatment nodes are 3 cm or smaller, patients undergo neck dissection if residual nodal disease is present.
During irradiation, inflammatory tissue and edema can be difficult to differentiate from residual tumor (15, 40). This is also problematic in the early posttreatment period, when immature scars and recurrent tumors can be indistinguishable radiographically (20). Although the entire area of abnormal T2 signal intensity (including tumor and edema) in the region of interest was outlined at all time points in our study, we found that pretreatment volume measurements had significantly less variability than measurements obtained during therapy (P = .01). Differences in reproducibility based on the stage of therapy were well demonstrated in one patient who had a percentage measurement error for a primary cancer of 6% before therapy and 53% during therapy. In our retrospective review of the images, we determined that the patient had such extensive treatment-induced changes that a completely different hyperintense region might have been measured on the follow-up study; this region could have contributed to the increased variability and introduced an outlier into our results. In addition, toward the end of successful treatment, a residual primary tumor or nodal mass may be small. In these situations, the absolute-difference volume measurements between readings can be small, and the percentage measurement error is potentially large (>20%) (Table 1).
Conclusions
We assessed intraobserver variability in MR imaging–based measurements of tumor volume by using an interactive computer program and found that these measurements are reproducible. Next, it would be important to look at the interobserver variability of volume measurements with more than one radiologist experienced in head and neck cancer. Tumor volumes may provide objective measures of the extent of neoplastic disease, which can then be used for assessing the prognosis or the response to therapy. Volumes may also be factored into therapeutic decision making, including the determination of when changes in therapy are necessary.
Footnotes
Supported by National Institutes of Health grant UOICA62559 and by the Radiological Society of North America Research and Education Foundation Scholar Grant.
References
- 1.National Cancer Institute 2001 FactBook. Bethesda: National Cancer Institute,2001
- 2.Gilbert RW, Birt D, Shulman H, et al. Correlation of tumor volume with local control in laryngeal carcinoma treated by radiotherapy. Ann Otol Rhinol Laryngol 1987;96:514–518 [DOI] [PubMed] [Google Scholar]
- 3.Freeman DE, Mancuso AA, Parsons JT, Mendenhall WM, Million RR. Irradiation alone for supraglottic larynx carcinoma: can CT findings predict treatment results? Int J Rad Oncol Biol Phys 1990;19:485–490 [DOI] [PubMed] [Google Scholar]
- 4.Lee WR, Mancuso AA, Saleh EM, Mendenhall WM, Parsons JT, Million RR. Can pretreatment computed tomography findings predict local control in T3 squamous cell carcinoma of the glottic larynx treated with radiotherapy alone? Int J Rad Oncol Biol Phys 1993;25:683–687 [DOI] [PubMed] [Google Scholar]
- 5.Castelijns JA, van den Brekel MWM, Smit EMT, et al. Predictive value of MR imaging-dependent and non-MR imaging-dependent parameters for recurrence of laryngeal cancer after radiation therapy. Radiology 1995;196:735–739 [DOI] [PubMed] [Google Scholar]
- 6.Pameijer FA, Mancuso AA, Mendenhall WM, et al. Evaluation of pretreatment computed tomography as a predictor of local control in T1/T2 pyriform sinus carcinoma treated with definitive radiotherapy. Head Neck 1998;20:159–168 [DOI] [PubMed] [Google Scholar]
- 7.Sakata K, Hareyama M, Tamakawa M, et al. Prognostic factors of nasopharynx tumors investigated by MR imaging and the value of MR imaging in the newly published TNM staging. Int J Rad Oncol Biol Phys 1999;43:273–278 [DOI] [PubMed] [Google Scholar]
- 8.Chua DTT, Sham JST, Kwong DLW, et al. Volumetric analysis of tumor extent in nasopharyngeal carcinoma and correlation with treatment outcome. Int J Rad Oncol Biol Phys 1997;39:711–719 [DOI] [PubMed] [Google Scholar]
- 9.Johnson CR, Thames HD, Huang DT, Schmidt-Ullrich RK. The tumor volume and clonogen number relationship: tumor control predictions based upon tumor volume estimates derived from computed tomography. Int J Rad Oncol Biol Phys 1995;33:281–287 [DOI] [PubMed] [Google Scholar]
- 10.Van den Bogaert W, van der Schueren E, Horiot JC, et al. The EORTC randomized trial on three fractions per day and misonidazole in advanced head and neck cancer: prognostic factors. Radiother Oncol 1995;35:100–106 [DOI] [PubMed] [Google Scholar]
- 11.Rudat V, Dietz A, Schramm O, et al. Prognostic impact of total tumor volume and hemoglobin concentration on the outcome of patients with advanced head and neck cancer after concomitant boost radiochemotherapy. Radiother Oncol 1999;53:119–125 [DOI] [PubMed] [Google Scholar]
- 12.Kawashima M, Ogino T, Fujii H, Ishikura S, Ito Y, Ikeda H. Local-regional control by conventional radiotherapy according to tumor volume in patients with squamous cell carcinoma of the pharyngolarynx. Jpn J Clin Oncol 1999;29:467–473 [DOI] [PubMed] [Google Scholar]
- 13.Mukherji SK, O’Brien SM, Gerstle RJ, Weissler M, Shckley W, Castillo M. Tumor volume: an independent predictor of outcome for laryngeal cancer. J Comput Assist Tomogr 1999;23:50–54 [DOI] [PubMed] [Google Scholar]
- 14.Mukherje SK, O’Brien SM, Gerstle RJ, et al. The ability of tumor volume to predict local control in surgically treated squamous cell carcinoma of the supraglottic larynx. Head Neck 2000;22:282–287 [DOI] [PubMed] [Google Scholar]
- 15.van der Brekel MWM, Castelijns JA, Snow GB. The role of modern imaging studies in staging and therapy of head and neck neoplasms. Semin Oncol 1994;21:340–348 [PubMed] [Google Scholar]
- 16.Zbaren P, Becker M, Lang H. Pretherapeutic staging of laryngeal carcinoma. Cancer 1996;77:1263–1273 [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000;92:205–216 [DOI] [PubMed] [Google Scholar]
- 18.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207–214 [DOI] [PubMed] [Google Scholar]
- 19.Leslie A, Fyfe E, Guest P, Goddard P, Kabala JE. Staging of squamous cell carcinoma of the oral cavity and oropharynx: a comparison of MRI and CT in T- and N-staging. J Comput Assist Tomogr 1999;23:43–49 [DOI] [PubMed] [Google Scholar]
- 20.Olmi P, Fallai C, Colagrande S, Giannardi G. Staging and follow-up of nasopharyngeal carcinoma: magnetic resonance imaging versus computerized tomography. Int J Rad Oncol Biol Phys 1995;32:795–800 [DOI] [PubMed] [Google Scholar]
- 21.Som PM, Shapiro MD, Biller HF, Sasaki C, Lawson W. Sinonasal tumors and inflammatory tissues: differentiation with MR imaging. Radiology 1988;167:803–808 [DOI] [PubMed] [Google Scholar]
- 22.Pameijer FA, Balm AJM, Hilgers FJM, Muller SH. Variability of tumor volumes in T3-staged head and neck tumors. Head Neck 1997;19:6–13 [DOI] [PubMed] [Google Scholar]
- 23.Pameijer FA, Mancuso AA, Mendenhall WM, Parsons JT, Kubilis PS. Can pretreatment computed tomography predict local control in T3 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy? Int J Rad Oncol Biol Phys 1997;37:1011–1021 [DOI] [PubMed] [Google Scholar]
- 24.Werber JL, Lucente FE. Computed tomography in patients with laryngeal carcinoma: a clinical perspective. Ann Otol Rhinol Laryngol 1989;98:55–58 [DOI] [PubMed] [Google Scholar]
- 25.Katsantonis GP, Archer CR, Rosenblum BN, Yeager VL, Friedman WH. The degree to which accuracy of preoperative staging of laryngeal carcinoma has been enhanced by computed tomography. Otolaryngol Head Neck Surg 1986;95:52—62 [DOI] [PubMed] [Google Scholar]
- 26.Silverman PM, Bossen EH, Fisher SR, Cole TB, Korobkin M, Halvorsen RA. Carcinoma of the larynx and hypopharynx: computed tomographic-histopathologic correlations. Radiology 1984;151:697–702 [DOI] [PubMed] [Google Scholar]
- 27.Giron J, Joffre P, Serres-Cousine O, Senac JP. CT and MR evaluation of laryngeal carcinomas. J Otolaryngol 1993;22:284–293 [PubMed] [Google Scholar]
- 28.Kabala J, Goddard P, Cook P. Magnetic resonance imaging of extracranial head and neck tumors. Br J Radiol 1992;65:375–383 [DOI] [PubMed] [Google Scholar]
- 29.O’Brien PC, Shampo MA. Statistics for clinicians: evaluating a new diagnostic procedure. Mayo Clin Proc 1981;56:573–575 [PubMed] [Google Scholar]
- 30.Gatsonis C, McNeil BJ. Collaborative evaluations of diagnostic tests: experience of the radiology diagnostic oncology group. Radiology 1990;175:571–575 [DOI] [PubMed] [Google Scholar]
- 31.Elliott MA, Walter GA, Gulish H, et al. Volumetric measurement of human calf muscle from magnetic resonance imaging. MAGMA 1997;5:93–98 [DOI] [PubMed] [Google Scholar]
- 32.Lydiatt DD, Markin RS, Williams SM, Davis LF, Yonkers AJ. Computed tomography and magnetic resonance imaging of cervical metastasis. Otolaryngol Head Neck Surg 1989;101:422–425 [DOI] [PubMed] [Google Scholar]
- 33.Grabenbauer GG, Steininger H, Meyer M, et al. Nodal CT density and total tumor volume as prognostic factors after radiation therapy of stage III/IV head and neck cancer. Radiother Oncol 1998;47:175–183 [DOI] [PubMed] [Google Scholar]
- 34.Correa AJ, Burkey BB. Current options in management of head and neck cancer patients. Med Clin North Am 1999;83:235–246 [DOI] [PubMed] [Google Scholar]
- 35.Willner J, Baier K, Pfreundner L, Flentje M. Tumor volume and local control in primary radiotherapy of nasopharyngeal carcinoma. Acta Oncol 1999;38:1025–1030 [DOI] [PubMed] [Google Scholar]
- 36.Van Hoe L, Van Cutsem E, Vergote I, et al. Size quantification of liver metastases in patients undergoing cancer treatment: reproducibility of one-, two-, and three-dimensional measurements determined with spiral CT. Radiology 1997;202:671–675 [DOI] [PubMed] [Google Scholar]
- 37.Hermans R, Feron M, Bellon E, Dupont P, Van den Bogaert W, Baert AL. Laryngeal tumor volume measurements determined with CT: a study on intra- and interobserver variability. Int J Rad Oncol Biol Phys 1998;40:553–557 [DOI] [PubMed] [Google Scholar]
- 38.Pattynama PMT, Lamb HJ, van der Velde EA, van der Wall EE, de Roos A. Left ventricular measurements with cine and spin-echo MR imaging: a study of reproducibility with variance component analysis. Radiology 1993;187:261–268 [DOI] [PubMed] [Google Scholar]
- 39.Rasch C, Keus R, Pameijer FA, et al. The potential impact of CT-MRI matching on tumor volume delineation in advanced head and neck cancer. Int J Rad Oncol Biol Phys 1997;39:841–848 [DOI] [PubMed] [Google Scholar]
- 40.Zbaren P, Becker M, Lang H. Staging of laryngeal cancer: endoscopy, computed tomography and magnetic resonance versus histopathology. Eur Arch Otorhinolaryngol 1997;254:S117–S122 [DOI] [PubMed] [Google Scholar]


