Abstract
Summary: We describe a fetus with agenesis of the corpus callosum (ACC) and Dandy-Walker malformation that developed a frontal paramidline cyst late in gestation. The interval appearance of the cyst occurred in concert with increasing size of the lateral ventricles, which supports the hypothesis that cysts associated with ACC can develop with increasing intraventricular pressure. Recognition of the potential for a changing appearance of neurologic abnormalities is important to providing appropriate patient counseling.
The origin of the interhemispheric cyst in agenesis of the corpus callosum (ACC) is controversial. Neurenteric, arachnoid, and ependymal cysts have all been suggested as possible causes. We present a case of Dandy-Walker malformation (DWM) and ACC wherein a cyst developed in the interval between prenatal imaging at 25 weeks and birth. The cyst developed in concert with increasing ventricular size, which suggests that the cyst is a form of communicating hydrocephalus and is an ependymal-lined cyst. This case illustrates the point that prenatal absence of an interhemispheric cyst does not necessarily mean that a cyst will not develop later in gestation.
Case Report
A 41-year-old woman, gravida 4, para 2, termination 1, had a sonogram at 16 weeks’ gestation at an outside institution. She reported being told that “something is wrong with the baby” at that time. The patient declined triple screen and amniocentesis and failed to seek further prenatal care until 25 weeks’ gestation, when she first came to our institution.
Sonography (Fig 1) at 25 weeks’ gestation demonstrated splayed cerebellar hemispheres with a large midline defect consistent with DWM. The lateral ventricles demonstrated colpocephaly with the anterior horns in a slitlike, parallel orientation, and distension of the occipital horns. The measurement of the cerebral ventricles at the atrium was 17 mm on the left and 15 mm on the right. These findings were consistent with ACC. Head size was 2 weeks greater than other biometric measurements. The patient underwent fetal MR imaging, which confirmed the diagnosis of DWM with ACC (Fig 2). The patient declined further prenatal imaging or genetic studies.
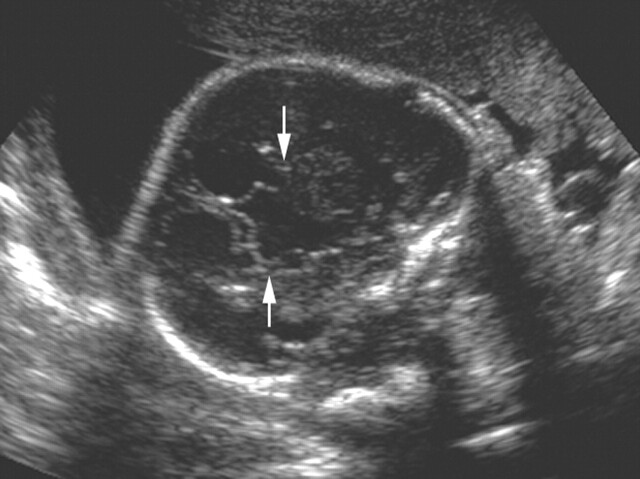
Fig 1.
Transabdominal sonogram obtained in a fetus with ACC and DWM at 27 weeks’ gestation. Coronal view shows elevation of the third ventricle, vertical orientation of the frontal horns (arrows), and absence of the corpus callosum.
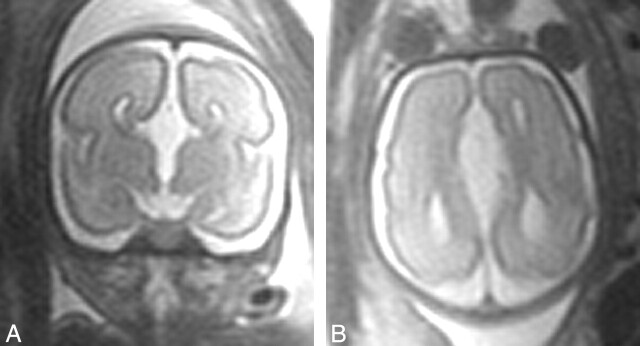
Fig 2.
Single-shot half-Fourier turbo spin-echo MR images of the fetal brain at 27 weeks’ gestation. Coronal (A) and axial (B) images demonstrate agenesis of the corpus callosum and absence of any frontal cyst.
At 38 weeks’ gestation, the patient presented with contractions and spontaneous membrane rupture. After 10 hours of labor, she underwent delivery by cesarean section because of arrest of cervical dilation, which was thought to be due to cephalopelvic disproportion. Uncomplicated surgery produced a 6-pound, 4-ounce baby boy with Apgar scores of 8 at 1 minute and 9 at 5 minutes. Head circumference was 36.5 cm (>95th percentile for gestational age).
In the 1st week of life, the infant underwent MR (Fig 3) and sonographic (Fig 4) imaging studies. They demonstrated the previously described ACC and DWM, but also showed a left frontal cyst. Retrospective review of prenatal MR images confirmed that this cyst had not been present at the time of the prenatal studies. Since the prenatal studies, the lateral ventricle size had increased to 26 mm on the left and 20 mm on the right. The falx cerebri was present but deviated by the frontal cyst. The frontal horns were now slightly prominent in size, although still small in comparison to the occipital horns.
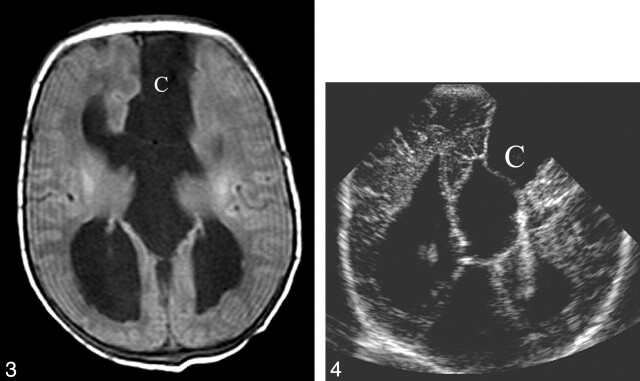
Fig 3.
Neonatal brain MR image taken when the patient was 1 day old. Axial T1-weighted image (similar plane to that of Figure 2B) shows the increased size of the frontal and occipital horns (compare with prenatal scan) in addition to the new finding of a frontal para-midline cyst (C).
Fig 4.
Neonatal cranial sonogram obtained when the patient was 5 days old. Coronal view in plane similar to that of Figures 2B and 3 demonstrates agenesis of the corpus callosum. In addition, a large frontal paramidline cyst is present (C).
Discussion
The association of both interhemispheric cysts and DWM with ACC is well recognized (1, 2). In 2001, Barkovich et al (3) presented a new system for classifying morphologically distinct cases of interhemispheric cysts that occur in the presence of ACC. This classification scheme grouped 25 cases on the basis of ventricular, cystic, and gross morphologic abnormalities, with the aim of associating types of cysts that may share elements of common origin and, possibly, prognosis. The various subtypes of cysts were classified with the assumption that the presence of a specific type of underlying cyst, ventricular defect, diencephalic malformation, or genetic anomaly produces malformations, deformations, or disruptions because of mass effect that may have similar imaging appearance. It is important to note that Barkovich et al (3) designed their classification system to rely on postnatal, not prenatal, imaging techniques and not to include pathologic or histologic diagnosis. The Barkovich classification divides cases of interhemispheric cysts associated with ACC into type 1 cysts, which are diverticula of the lateral or third ventricles, and type 2 cysts, which are loculated and do not appear to communicate with the ventricular system (Table).
Classification of agenesis of the corpus callosum with interhemispheric cyst after Barkovich et al (3)
| Type 1* | |||
|---|---|---|---|
| Subtype | Cyst Characteristics | Communication | Associated Abnormalities |
| Type 1a: Presumed communicating hydrocephalus | Isointense to CSF (MR), unilocular | Communication with lateral ventricles only | Macrocephaly, hydrocephalus, Dandy-Walker malformation |
| Type 1b: Hydrocephalus secondary to diencephalic anomaly | Isointense to CSF (MR), unilocular | Communication with and obstruction of third ventricle | Macrocephaly, diencephalic malformation (eg, thalamic fusion without subcortical heterotopia) |
| Type 1c: Small head size and cerebral hypoplasia | Isointense to CSF (MR), unilocular | Communication with lateral and third ventricles | Microcephaly, cerebral dysplasia or hypoplasia |
| Type 2† | |||
| Type 2a: No abnormality apart from ACC | Isointense to CSF (MR), multilocular | No communication with lateral or third ventricles | Macrocephaly, hydrocephalus |
| Type 2b: Aicardi syndrome | Hyperattenuation (CT), hyperintense (T1W MR), multilocular | No communication with lateral or third ventricles | Female predominance, subependymal heterotopia, polymicrogyria, seizures, hypoplastic falx cerebri, uni- or bilateral ventriculomegaly, developmental delay |
| Type 2c: Subcortical heterotopia | Isointense to CSF (MR), multilocular | No communication with lateral or third ventricles | Subcortical heterotopia, developmental delay |
Extension or diverticulation of third or lateral ventricles.
Loculated, lack of communication with ventricular system.
In some cases, classification of congenital intracranial cysts can be performed on the basis of autopsy and surgical specimens. Although definitive correlation of intracranial imaging of a cyst and histologic type has not been completed to date, characteristics of intracranial cysts seen at sonography and MR imaging can lead to a presumptive diagnosis of cyst histology. On the basis of their location, morphology, and imaging characteristics, intracranial cysts associated with ACC are most likely arachnoid, neurenteric, or ependymal in origin.
Arachnoid cysts are thought to develop secondary to splitting or duplication of normal arachnoid membrane during development. Most are diagnosed in childhood, with 50% reported before the age of 5 years (4). Arachnoid cysts are considered benign, although compression of adjacent structures can have considerable pathologic consequences if the cyst is large in size. Arachnoid cysts are thought to develop and expand by one of three mechanisms: the ball-valve hypothesis, in which communication of the cystic malformation with the subarachnoid space allows for CSF flow into but not out of the cyst; fluid production by the cells of the cyst wall itself; and the presence of an osmotic gradient drawing CSF into the cyst. Arachnoid cysts can be distinguished from other types of intracranial cysts at MR imaging on the basis of location (generally extraaxial), morphology of the cyst (including visualization of a characteristic surrounding collagen layer and the absence of traversing trabeculations), and signal intensity matching that of CSF (5).
Unlike arachnoid cysts, ependymal cysts are most commonly found deep in the brain parenchyma, although subarachnoid and intraventricular (noncommunicating) ependymal cysts have been reported (6). By light microscopy, histologic sections of the cyst wall reveal a single layer of columnar brush-border cells with microvilli consistent with ependymal cells abutting white matter (6, 7). Like arachnoid cysts, ependymal cysts are benign but have pathologic consequences owing to mass effect. They are generally slowly progressive, expanding cysts generated by a collection of ependymal cell secretions (8).
Neurenteric cysts derive from maldevelopment or persistence of the neuroenteric canal (8). On histologic examination, a variety of nonciliated cell types are present, many with obvious secretory capacity in the form of secretory granules or neurosecretory vesicles or both (7). Mesodermal tissue derivatives such as muscle, fat, or cartilage cells may also be present (8). On MR imaging studies, these cysts appear smooth and unilocular with proteinaceous content of different signal intensity than that of CSF (9) and are most often located extracranially (8).
According to the Barkovich et al classification, this case is an example of type 1a cyst, meaning it is not loculated, and therefore is presumably an outpouching of the lateral ventricle and does not appear to result from a diencephalic malformation blocking the aqueduct of Sylvius. Like the type 1a cases described by Barkovich et al, this case demonstrated increasing hydrocephalus and macrocephaly in concert with appearance of the interhemispheric cyst. This supports the theory that the type 1 interhemispheric cysts are caused by obstruction of normal CSF circulation. It also suggests that type 1 cysts such as that seen in this case are ependymal cysts.
In this report, we describe a frontal paramidline cyst that developed in the setting of ACC and DWM after the completion of prenatal imaging. This case emphasizes the fact that, even when ACC is identified in prenatal screening, accompanying pathologic abnormality may not be present at the time of prenatal imaging. This finding is consistent with the report of a French study that saw 45% of intracranial cysts develop after the 30th week of gestation (10). Because prenatal imaging is usually completed during the second trimester in the United States, it is especially important to recognize the potential for a changing appearance of intracranial abnormalities during the third trimester. This concept is important, because the presence of such a cyst may change counseling of the patient, although management would likely be affected by ventricular size or mass effect of the cyst rather than solely by the presence of a cyst.
Footnotes
A.S.S. and D.L. are supported by the Carl J. Shapiro Institute for Education and Research Educational Innovation Fund. D.L. is also supported by the National Institutes of Health grant NS37945.
References
- 1.Probst FP. Agenesis of the corpus callosum. Acta Radiol 1973;331(Suppl):1–152 [PubMed] [Google Scholar]
- 2.Brihaye J, Gilet P, Parmentier R, Peetrons E. Agenesie de la commissure calleuse associe ave un kyst ependymaire. Schwietz Arch Neurol Psychiatr 1956;77:615–631. [PubMed] [Google Scholar]
- 3.Barkovich AJ, Simon EM, Walsh CA. Callosal agenesis with cyst: a better understanding and new classification. Neurology 2001;56:220–227 [DOI] [PubMed] [Google Scholar]
- 4.Dodd RL, Barnes PD, Huhn SL. Spontaneous resolution of a prepontine arachnoid cyst. Pediatr Neurosurg 2002;37:152–157 [DOI] [PubMed] [Google Scholar]
- 5.Gosalakkal JA. Intracranial arachnoid cysts in children: a review of pathogenesis, clinical features, and management. Pediatr Neurol 2002;26:93–98 [DOI] [PubMed] [Google Scholar]
- 6.Pawar SJ, Sharma RR, Mahapatra AK, Dev EJ. Giant ependymal cyst of the temporal horn: an unusual presentation. Pediatr Neurosurg 2001;34:306–310 [DOI] [PubMed] [Google Scholar]
- 7.Hirano A, Hirano M. Benign cystic lesions in the central nervous system. Childs Nerv Syst 1988;4:325–333 [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Nayak SR, Krishani N, Chabra DK. Spinal intramedullary ependymal cyst. Pediatr Neurosurg 2001;35:29–34 [DOI] [PubMed] [Google Scholar]
- 9.Evans A, Stoodley N, Halpin S. Magnetic resonance imaging of intraspinal cystic lesions: a pictoral review. Curr Probl Diagn Radiol 2002;31:79–94 [DOI] [PubMed] [Google Scholar]
- 10.Pierre-Kahn A, Hanlo P, Sonigo P, et al. The contribution of prenatal diagnosis to the understanding of malformative intracranial cysts: state of the art. Childs Nerv Syst 2000;16:618–626 [DOI] [PubMed] [Google Scholar]