Abstract
SUMMARY: CT and MR imaging showed diffuse changes of the frontal white matter and genu of the corpus callosum with minimal atrophy and no contrast enhancement in a 41-year-old woman with progressive dementia. Brain biopsy disclosed axonal spheroids and gliosis in the white matter without macrophage or inflammatory infiltration or vessel abnormalities consistent with neuroaxonal leukodystrophy. This disease can be suspected on CT and MR imaging findings but requires neuropathologic examination to be diagnosed.
CT and MR imaging evidence in an adult of white matter damage confined to the frontal lobes and anterior corpus callosum with mild atrophy and without abnormal contrast enhancement is distinctly uncommon and represents a diagnostic challenge. In fact, the white matter damage in leukodystrophies with adult onset as late-onset metachromatic leukodystrophy and globoid cell leukodystrophy usually is not confined to the frontal lobes.1,2 An exclusive frontal distribution can be observed in adrenoleukodystrophy3 and adult-onset Alexander disease,4,5 but in the former condition contrast enhancement adjacent to the affected area and brain stem lesions are often present, whereas in the latter condition white matter changes are generally accompanied by atrophy of the medulla and spinal cord.4,5 Also other sporadic and inherited leukoencephalopathies of adults, including progressive subcortical gliosis,6 Binswanger disease,7 cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy,8 and vanishing white matter disease,9 are not confined to the frontal lobes, show a patchy distribution, or exhibit a more destructive type of brain damage with formation of lacunes and cysts. Finally, in frontotemporal dementia, the white matter changes are mild and accompanied by severe atrophy of the frontal and temporal lobes.10
Neuroaxonal leukodystrophy (NAL), also termed leukoencephalopthy with axonal spheroids, is a rare cause of sporadic or inherited early-onset dementia with white matter changes.11–16 We report a case of sporadic NAL with an exclusive frontal distribution of CT and MR imaging changes.
Case Report
A 41-year-old woman without history of familial neurologic diseases, alcohol abuse, or exposure to environmental toxins was admitted to the hospital because her mother had noted a progressive personality change since the patient was 34 years of age, when she became childish, impulsive, inattentive, and violent. Moreover, she had difficulties at work, had lost care of her personal hygiene, and had gained 10 kg of weight within 1 year because of compulsive eating. The neurologic examination revealed ataxia in the tandem walk, mild right hemiparesis with Babinski sign, diffusely increased deep tendon reflexes, dysmethria in the index-to-nose and heel-to-knee tasks, and horizontal nystagmus. Routine blood and urine laboratory examinations were normal. Electroencephalography showed diffuse slowing. Cranial CT (Fig 1) showed hypoattenuated areas in the frontal white matter and the genu of corpus callosum without mass effect or contrast enhancement. MR imaging (Fig 1) confirmed symmetric areas of low signal intensity in T1-weighted images and high signal intensity in proton attenuation and T2-weighted images involving the entire subcortical frontal white matter and the head and genu of the corpus callosum. No contrast enhancement was seen. Brain single-photon emission tomography with hexamethylpropyleneamine oxime (HMPAO) was normal. The activity of lysosomal enzymes in the serum and leukocytes, including β-esosaminidase, arysulfatase A, α-mannosidase, β-galactosidase, β-glucuronidase, α-fucosidase, α-galactosidase, and β-glucosidase, as well as the serum level of very long-chain fatty acids, were normal.
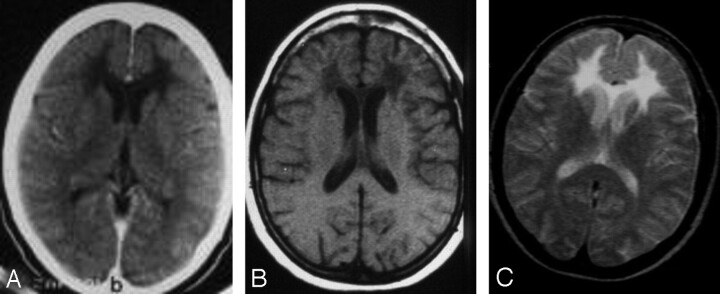
Fig 1.
Axial contrast-enhanced CT scan (A); unenhanced axial T1-weighted (B) MR image (repetition time, 460 milliseconds; echo time, 20 milliseconds; number of excitations, 4 [TR/TE/NEX]), and T2-weighted (C) MR image (2000/100/2) show low attenuation and signal intensity changes confined to the frontal white matter and the genu of the corpus callosum. No contrast enhancement or overt atrophy is present.
CSF examination was unremarkable. Neuropsychologic testing revealed marked deficit of attention and of long-term verbal memory with sparing of immediate memory and verbal fluency. Stereotactic brain biopsy of the right frontal lobe was carried out. Microscopic examination (Fig 2) showed reactive astrocytosis and axonal dilations (spheroids) in the subcortical white matter. The spheroids were argyrophilic and strongly stained with antibodies to neurofilaments (Fig 2) but were negative for tau and ubiquitin. No demyelination or lymphocytic infiltrates were observed. The cortical and medullary vessels were unremarkable. The cerebral cortex showed only mild gliosis. Ultrastructural examination of the affected white matter (Fig 2) revealed swelling of amyelinated and myelinated axons, whose cytoplasms were filled with altered mithocondria, vesicles, attenuated bodies, neurofilaments, and microtubules. In some axons, the cytoskeletal proteins were displaced toward the periphery, whereas mithocondria and attenuated bodies tended to occupy the central area.
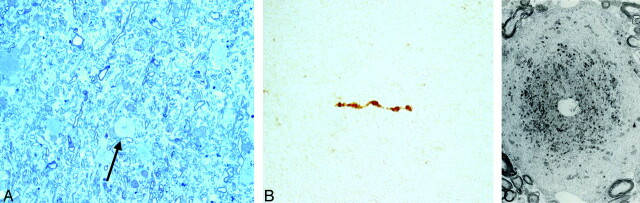
Fig 2.
A, Tolouidine blue staining (60×) of the stereotactic biopsy of the right frontal subcortical white matter shows numerous astrocytes and myelinated fibers and an axonal dilation spheroid (arrow). B, Paraffin section of the affected white matter (15×) stained with antibodies to neurofilaments (DAKO) demonstrates intense staining within a spheroid. C, Ultrastructural examination (8000×) of the affected white matter shows a swollen axon whose cytoplasm is filled in the central area with altered mithocondria and attenuated bodies, whereas neurofilaments and neurotubules tend to occupy the periphery.
One year later, the patient’s neurologic examination was unchanged but her mental deterioration had progressed, with alternating periods of apathy and hyperexcitability. MR imaging at age 43 showed mild extension of the areas of signal intensity changes to the anterior arm of the internal capsule bilaterally with minimal cortical atrophy and no abnormal contrast enhancement. Two years later, the patient had a generalized seizure. Electroencephalography showed a diffuse severe alteration of the background activity. The patient was transferred to a nursing home, where she became unresponsive and finally mute. She died at 46 years of age. Autopsy was not performed.
Discussion
Axonal spheroids are eosinophilic axonal swellings that are commonly observed in the gray matter in a number of conditions such as degenerative and metabolic diseases, intoxication, trauma, tumors and neuroaxonal dystrophies,14 including Seitelberg disease; late infantile, juvenile, and adult neuroaxonal dystrophies; Hallervorden-Spatz disease; and Nasu-Hakola disease. When axonal spheroids are confined to the white matter, the differential diagnosis is circumscribed to 3 entities: NAL, membranous lipodystrophy, and dermatoleukodystrophy with neuroaxonal spheroids.14 Whereas NAL is a disease of the adult, membranous lipodystrophy is a rapidly fatal condition of the small child and dermatoleukodystrophy with neuroaxonal spheroids is a disease of bone combined with leukoencephalopathy and calcifications of the basal ganglia.14 Although the ancillary clinical features and the negative results of laboratory tests enabled us to rule out several of the leukodystrophies and leukoencephalopathies with an adult onset, only histopathology made possible a diagnosis of NAL in our patient. The latter, as in one previously reported case,14 was accomplished by stereotactic biopsy. In fact, the neuropathologic findings in our patient were fundamentally identical to those in other reported cases of NAL.11–16 These consist of astrocytosis and axonal spheroids confined to the white matter without evidence of lymphocyte infiltrations or inclusion bodies. The cortical gray matter is typically spared. Ultrastructural examination shows accumulation of cytoskeletal proteins, in particular neurofilaments, and mithocondria in the dilated axons.14,15 In cases with a relatively short disease duration (21 and 27 months)15,16 overt demyelination and reactive macrophages containing myelin debris were observed. We submit that the mild demyelination (evident only at the ultrastructural examination) and lack of macrophages in our case might be related to the slow progression of the pathologic process as indicated by the long interval between onset and death (12 years). Dense bodies within the dilated axons were previously reported.15
The clinical features of NAL are rather homogeneous and in our patient were consistent with previous reports.11,13,14,16 Familial cases exhibit autosomal dominant inheritance,11,13,14,16 but no genetic abnormality has been identified so far. A high incidence of de novo mutations was proposed to explain the apparently sporadic cases.14 The disease onset ranges between 15 and 65 years of age11,14 but most often it occurs in the 20–50-year age interval. The disease duration from onset to death ranges between 21 months and 17 years.14,16 Presenting symptoms include mental deterioration with frontal features and seizures, which are combined in advanced phases with bilateral but asymmetric pyramidal syndrome.
The descriptions of the gross pathologic findings in autoptic cases and the CT and MR imaging features in the few available reports11–16 indicate that NAL typically determines bilateral but asymmetric white matter damage in the frontal-parietal regions that extends caudally along the internal capsules to the pons. The cortex and the basal ganglia are spared. The exclusive and symmetric distribution of damage to the frontal lobes in our case is atypical, and, although longitudinal evaluation of our patient was limited, we submit that it may justify its consideration as a frontal variant. Moreover, we emphasize the involvement of the genu of the corpus callosum in our patient. This was previously reported in 2 other cases.14,15 The nonspecific hypoattenuation on CT and hyperintensity on proton attenuation and T2-weighted MR images of the affected white matter in NAL presumably reflect the variable combination of gliosis and demyelination observed at the histopathologic examination. No contrast enhancement of the affected white matter was reported so far. It is noteworthy that the gross distribution of findings—namely, the exclusive white matter involvement—differentiates NAL from dementia with neurofilament inclusion,17 which may show some similarity from the neuropathologic point of view but is a predominantly gray matter disease, according to the available pathologic and MR imaging descriptions.
As for other leukoencephalopathies, the possible contribution of CT and MR imaging to the in vivo identification of the white matter changes in NAL is remarkable, but the differential diagnosis based on the CT and MR imaging features alone is very difficult. This is because of the nonspecificity of the CT attenuation or MR imaging signal intensity changes, the limited specificity of the distribution patterns,1,2 and, as exemplified by our case, by the possible occurrence of distribution variants.
In conclusion, we report an additional sporadic case of NAL with an exclusive distribution of changes in the frontal lobes and confirm that the diagnosis of this condition requires neuropathologic examination.
References
- 1.Barkovich AJ. Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol 2000;21:1099–109 [PMC free article] [PubMed] [Google Scholar]
- 2.Farina L, Bizzi A, Finocchiaro G, et al. MR imaging and proton MR spectroscopy in adult Krabbe disease. AJNR Am J Neuroradiol 2000;21:1478–82 [PMC free article] [PubMed] [Google Scholar]
- 3.Castellotte A, Vera J, Vazquez E, et al. MR in X-adrenoleukodystrophy: atypical presentation as bilateral frontal demyelination. AJNR Am J Neuroradiol 1995;16:814–15 [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto Y, Mitsuyama H, Jonosono M, et al. Autosomal dominant palatal myoclonus and spinal cord atrophy. J Neurol Sci 2002;195:71–76 [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita T, Imaizumi T, Miura Y, et al. A case of adult-onset Alexander disease with Arg416Trp human glial fibrillary acidic protein gene mutation. Neurosci Lett 2003;350:169–72 [DOI] [PubMed] [Google Scholar]
- 6.Vermersch P, Daes-Monpeurt C, Parent M, et al. Subcortical dementia of the Neumann type: contribution of diagnostic imaging. Rev Neurol 1994;150:354–58 [PubMed] [Google Scholar]
- 7.Mascalchi M, Inzitari D, Dal Pozzo G, et al. Computed tomography, magnetic resonance imaging and pathological correlations in a case of Binswanger’s disease. Can J Neurol Sci 1989;16:214–18 [DOI] [PubMed] [Google Scholar]
- 8.Sabbadini G, Francia A, Calandriello L, et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Brain 1995;118:207–15 [DOI] [PubMed] [Google Scholar]
- 9.Prass K, Bruck W, Schroder NWJ, et al. Adult-onset leukoencephalopathy with vanishing white matter presenting with dementia. Ann Neurol 2001;50:665–68 [DOI] [PubMed] [Google Scholar]
- 10.Savoiardo M, Grisoli M. Imaging dementias. Eur Radiol 2001;11:484–92 [DOI] [PubMed] [Google Scholar]
- 11.Axelsson R, Roytta M, Sourander P, et al. Hereditary diffuse leukoencephalopathy with spheroids. Acta Psychiatr Scand 1984;69(suppl 314):7–65 [PubMed] [Google Scholar]
- 12.Goodman LA, Dickson DW. Non hereditary diffuse leukoencephalopathy with spheroids presenting as early-onset rapidly progressive dementia. J Neuropathol Exp Neurol 1995;54:471 [Google Scholar]
- 13.Yazawa I, Nakano I, Yamada H, et al. Long tract degeneration in familial leukodistrophy with prominent spheroids. J Neurol Sci 1997;147:185–91 [DOI] [PubMed] [Google Scholar]
- 14.van der Knaap MS, Naidu S, Kleinshmidt-DeMasters BK, et al. Autosomal dominant diffuse leukoencephalopathy with axonal spheroids. Neurology 2000;54:463–68 [DOI] [PubMed] [Google Scholar]
- 15.Yamashita M, Yamamoto T. Neuroaxonal leukoencephalopathy with axonal spheroids. Eur Neurol 2002;48:20–25 [DOI] [PubMed] [Google Scholar]
- 16.Hancock N, Poon M, Taylor B, et al. Hereditary diffuse leucoencephalopathy with spheroids. J Neurol Neurosurg Psychiatry 2003;74:1335–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Josephs KA, Holton JL, Rossor M, et al. Neurofilament inclusion body disease: a new proteinopathy? Brain 2003;126:2291–303 [DOI] [PubMed] [Google Scholar]