Abstract
Summary: Wernicke encephalopathy is caused by thiamine deficiency. Although the clinical picture has been well established for some time, clinical diagnosis is attained in only 20% of the cases. MR imaging techniques contribute to early diagnosis of Wernicke encephalopathy. We herein report MR imaging and proton MR spectroscopic findings for a patient with clinical and biochemical features consistent with Wernicke encephalopathy. Increased lactate and typical MR imaging findings are discussed in the context of the known pathophysiology of Wernicke encephalopathy.
Wernicke encephalopathy is a metabolic disorder caused by thiamine (B1 vitamin) deficiency and is classically characterized by the clinical triad of ocular movement abnormalities, ataxia, and confusional state. Its prevalence in various neuropathologic studies is variable (nearly 2.8%) (1), and its incidence is reported to be 1.7% (2). Despite this notably high frequency, clinical diagnosis of Wernicke encephalopathy is made in only 20% of the cases (3). It is important to understand that Wernicke encephalopathy has been described as being associated with many different non-alcohol-related pathologic conditions that share the common denominator of causing malnutrition and include hematologic malignancies (4).
During recent years, MR imaging has proved to be very useful in confirming the diagnosis of Wernicke encephalopathy and in contributing to earlier detection (5). Recently, reports of patients with Wernicke encephalopathy have shown restricted diffusion on diffusion-weighted images of brain regions characteristically compromised in this entity (6, 7). Only one description of proton MR spectroscopic changes in a patient with Wernicke encephalopathy has been reported (8). Even though the changes shown by diffusion-weighted imaging and especially proton MR spectroscopy have not yet been fully understood, they seem to constitute a new tool for understanding and diagnosing Wernicke encephalopathy. We herein report the case of a patient with reversible Wernicke encephalopathy who developed cerebral lesions and had changes shown by diffusion-weighted imaging and proton MR spectroscopy.
Case Report
A 34-year-old woman with acute myeloid leukemia was admitted to our center in July 2000 because of persisting nausea and vomiting associated with significant weight loss. Two months earlier, she had undergone bone marrow transplantation, and a few days later, she developed appetite loss, nausea, and vomiting that were initially attributed to chemotherapy. The symptoms did not resolve and were proved to be refractory to any medical treatment, so the patient was hospitalized.
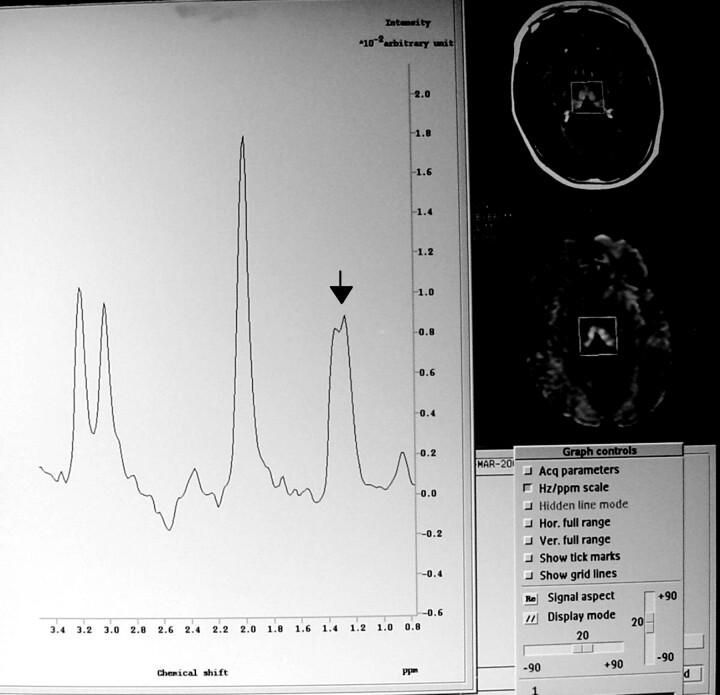
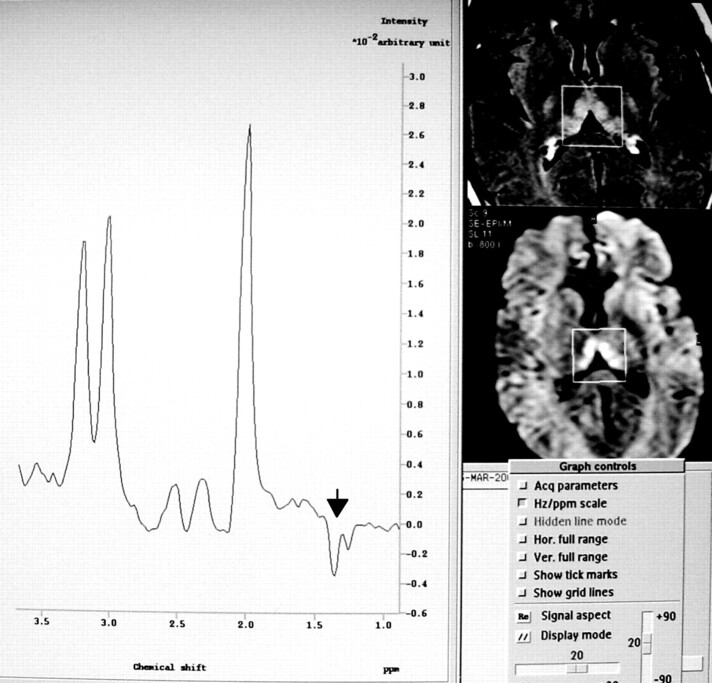
At admission, she was alert and well orientated without any neurologic abnormality. Because of oral intolerance, she was treated with IV administered dextrose saline solutions without vitamin supplementation. Blood examinations showed only moderate elevation of pancreatic enzyme concentration. The results of sonography and contrast-enhanced CT of the abdomen were normal, and an upper gastrointestinal tract videoendoscopy revealed a mild superficial gastritis. The findings of contrast-enhanced MR imaging of the brain performed 2 days after admission were normal. During the ensuing 5 days, the patient’s condition evolved with progressive frontal cephalea and dizziness. A neurologic examination showed mild inattention, central nystagmus, and axial ataxia. New laboratory tests did not reveal any significant data. The results of CT of the brain were normal. CSF examination revealed 2 cells/mm3 normal protein and low glucose concentration (28 mg/dL). Blood and CSF cultures were negative for bacterial or fungal infection, and the results of HIV and Venereal Disease Research Laboratories tests were also negative. A bone marrow biopsy ruled out acute myeloid leukemia relapse. The patient’s condition rapidly worsened, adding diplopia, lethargy, confusion, appendicular ataxia, and mild generalized weakness associated with decreased deep tendon reflexes. A second CSF examination revealed 2 cells/mm3, normal glucose (71 mg/dL), and elevated protein (57 mg/dL) concentration with an elevation of lactate to 50 mg/dL (normal range, 5.7–22 mg/dL). Cytomegalovirus serology and CSF protein chain reaction for tuberculosis and herpes virus were negative. Repeat MR imaging of the brain performed 10 days after admission and 6 days after the beginning of neurologic symptoms disclosed hyperintensities on T2-weighted, fluid-attenuated inversion recovery, and diffusion-weighted images in both dorsal and medial nuclei of the thalamus, periaqueductal gray matter, and superior and anterior cerebellar vermis without contrast enhancement or mass effect. The diffusion-weighted imaging abnormalities correlated with decreased signal intensity in the same areas on apparent diffusion coefficient maps, indicating restrictive diffusion. Two single voxel proton spectroscopic sequences with point-resolved spectroscopy volume selection (Philips) were measured with 2000/136, 272 (TR/TE, second TE). A total of 128 measurements were averaged, yielding an imaging time of 4 min 24 s, with 512 data points. Water suppression was achieved with an inversion pulse. A cubic 8-mL voxel was localized in the region of the T2 hyperintensity in the diencephalon, which showed a lactate doublet with normal N-acetylaspartate:creatine and N-acetylaspartate:choline ratios (Figs 1 and 2).
Fig 1.
Proton MR spectroscopic image (TE, 272) shows a positive doublet centered at 1.3 ppm without reduction of N-acetylaspartate.
Fig 2.
Proton MR spectroscopic image (TE, 136) confirms lactate as a negative doublet at this TE.
On the basis of these clinical and imaging features, a possible Wernicke encephalopathy diagnosis was made. After obtaining a blood sample of thiamine concentration, IV administration of this vitamin was initiated at doses of 100 mg/day. The serum level of thiamine pretreatment was decreased: 1.10 ng/mL (normal range 1.5–5.5 ng/mL). The patient’s condition rapidly improved, and the results of a neurologic examination performed 5 days after thiamine reposition were normal, with the exception of a mild horizontal and bidirectional nystagmus that resolved 1 month later.
Discussion
This patient presented with a classical acute neurologic picture for Wernicke encephalopathy. MR imaging of the brain showed the characteristic lesions distribution for Wernicke encephalopathy: the dorsal and medial nuclei of both thalami, periaqueductal gray matter, and cerebellar vermis (3, 9).
The mechanism by which thiamine deficiency causes the focal neuropathologic lesions described in association with Wernicke encephalopathy might be multiple (10). Thiamine acts as a coenzyme of three critical enzymes in the intermediate carbohydrate metabolism: transketolase, α-ketoglutarate dehydrogenase, and pyruvate dehydrogenase complex. The transketolase is involved in redox equilibrium, nucleic acid synthesis, and maintenance of membrane phospholipid integrity (10). The decreased cerebral α-ketoglutarate dehydrogenase activity induces a decrease in adenosine 5′-triphosphate production in vulnerable regions, leading to an increase of lactate levels and neuronal excitotoxicity. All these biochemical changes promote cell death by necrosis and apoptosis (10). They lead to the histologic lesions widely described in previous reports (2), including intra- and extracellular edema and proliferation of pleomorphic microglia as the earliest modification. Demyelination and vascular changes then become prominent in the subacute stage, with hemorrhagic lesions occurring in some cases. Finally, loss of parenchymal elements was noted to occur during the chronic stages, particularly in the thalami.
The clinical evolution and the symmetrical hyperintensities disclosed by T2-weighted, fluid-attenuated inversion recovery, and diffusion-weighted imaging suggest an acute stage of the disease in this patient, considering that this hyperintense signal intensity has recently been correlated with edema by some authors, especially when it is seen on diffusion-weighted images (6, 7). The apparent diffusion coefficient for our patient was decreased, which is a finding that has been associated with cytotoxic edema (6, 7). The neurologic symptoms in our patient reversed completely, and no sequelae remained. In this case, the decreased apparent diffusion coefficient was visually determined, and we did not have the opportunity of calculating the apparent diffusion coefficient value.
Proton MR spectroscopy performed during the acute stage of the disease showed a remarkable increase in lactate as an isolated abnormality. This elevation was also detected by the second CSF analysis. By using an 8-mL MR spectroscopic voxel centered at the thalami, the acquired signal intensity probably averaged contributions from CSF in the third ventricle and cisternal neighboring spaces. Nevertheless, it does not invalidate the diagnosis of increased anaerobic metabolism because lactate produced in the brain diffuses to CSF, where CSF is not normally detected. The calculation of metabolite ratios theoretically accounts for CSF contamination. Because both terms (ie, numerator and denominator) are essentially equally contaminated with CSF (neglecting chemical shift effect), the ratio value is therefore “atrophy corrected.”
To date, there have been very few reports of proton MR spectroscopy in cases of Wernicke encephalopathy (8, 11, 12). Lee et al (11) described a significant decrease in cerebral concentration of choline-containing compounds in the thiamine-deficient rat brain that presented a quick dose-dependent recovery with thiamine replenishment. The authors attributed this finding to a reduced incorporation of lipids into myelin or reduced synthesis of brain acetylcholine. The brain choline concentration was normal in our patient. Murata et al (8) reported the only case of Wernicke encephalopathy in a human who underwent proton MR spectroscopy. That patient had MR imaging signal intensity abnormalities located in the cerebellum that did not reverse after thiamine treatment. Proton MR spectroscopy in that case disclosed low levels of N-acetylaspartate:creatine in the thalamus and cerebellum and a lactate peak in the cerebellum that resolved partially. Therefore, the authors assumed that irreversible necrosis might have occurred. Our patient did not present with decreased N-acetylaspartate:creatine, but she did present with a significant lactate peak localized in the thalami. We speculate that the complete clinical resolution in this case suggests that the lactate peak detected was not secondary to local necrosis but to the increased anaerobic oxidation of carbohydrates associated with thiamine deficiency, exaggerated by the parenteral dextrose load that the patient received during the first days of hospitalization. This pathophysiological mechanism was well studied by Rose et al (12) who found a lactate peak by performing localized in vivo proton MR spectroscopy of rat brains with experimental deficiency of thiamine and glucose loading.
In short, Wernicke encephalopathy is a potentially reversible condition with high morbidity and mortality rates that usually depend on the delay in starting thiamine treatment. Because the classic clinical triad is present in only 16% of cases (3), other paraclinical methods have become extremely important. The combination of conventional MR imaging with some of its new developments, such as diffusion-weighed imaging and proton MR spectroscopy, seems to constitute a powerful diagnostic tool with potentially prognostic value.
References
- 1.Harper C, Fornes P, Duyckaerts C, Lecomte D Hauw JJ. An international perspective on the prevalence of Wernicke-Korsakoff syndrome.Metab Brain Dis 1995;10:17–24 [DOI] [PubMed] [Google Scholar]
- 2.Harper C. Wernicke’s encephalopathy: a more common disease than realized.J Neurol Neurosurg Psychiatry 1979;42:226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy.J Neurol Neurosurg Psychiatry 1986;49:341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pihko H, Saarinen U, Paetau A. Wernicke encephalopathy: a preventable cause of death: report of 2 children with malignant disease.Pediatr Neurol 1989;5:237–242 [DOI] [PubMed] [Google Scholar]
- 5.Antunez E, Estruch R, Cardenal C, Nicolas JM, Fernandez-Sola J, Urbano-Marquez A. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke’s encephalopathy.AJR Am J Roentgenol 1998;171:1131–1137 [DOI] [PubMed] [Google Scholar]
- 6.Doherty MJ, Watson NF, Uchino K, Hallam DK, Cramer SC. Diffusion abnormalities in patients with Wernicke encephalopathy.Neurology 2002;58:655–657 [DOI] [PubMed] [Google Scholar]
- 7.Chu K, Kang DW, Kim HJ, et al. Diffusion-weighted imaging abnormalities in wernicke encephalopathy: reversible cytotoxic edema?Arch Neurol 2002;59:123–127 [DOI] [PubMed] [Google Scholar]
- 8.Murata T, Fujito T, Kimura H, Omori M, Itoh H, Wada Y. Serial MRI and (1)H-MRS of Wernicke’s encephalopathy: report of a case with remarkable cerebellar lesions on MRI [abstr].Psychiatry Res 2001;108:49–55 [DOI] [PubMed] [Google Scholar]
- 9.Bae SJ, Lee HK, Lee JH, Choi CG, Suh DC. Wernicke’s encephalopathy: atypical manifestation at MR imaging.AJNR Am J Neuroradiol 2001;22:1480–1482 [PMC free article] [PubMed] [Google Scholar]
- 10.Hazell AS, Todd KG, Butterworth RF. Mechanism of neuronal cell death in Wernicke’s encephalopathy.Metab Brain Dis 1998;13:97–122 [DOI] [PubMed] [Google Scholar]
- 11.Lee H, Tarter J, Holburn GE, Price RR, Weinstein DD, Martin PR. In vivo localized proton NMR spectroscopy of thiamine deficient rat brain.Magn Reson Med 1995;34:313–318 [DOI] [PubMed] [Google Scholar]
- 12.Rose SE, Nixon PF, Zelaya FO, et al. Application of high field localised in vivo 1H MRS to study biochemical changes in the thiamin deficient rat brain under glucose load.NMR Biomed 1993;6:324–328 [DOI] [PubMed] [Google Scholar]