Abstract
Summary: We present the case of a patient with an MR imaging study showing an ill-defined intra-axial mass in the right insula and frontal lobe. The mass showed high signal intensity on T2-weighted and fluid-attenuated inversion recovery images and an unusual proton MR spectroscopic imaging pattern characterized by the presence of high levels of myo-inositol/glycine, no significant elevation of choline, and mildly reduced N-acetylaspartate. The histopathologic diagnosis was of diffuse astrocytoma with oligodendroglial components (World Health Organization grade II).
Low grade diffuse astrocytomas are histologically well-differentiated tumors distinguished by slow growth, diffuse infiltration of the surrounding brain, and a tendency to progress to more malignant types, such as anaplastic astrocytoma (World Health Organization grade III) and glioblastoma multiforme (World Health Organization grade IV). Proton MR spectroscopy of low grade astrocytomas shows elevation of myo-inositol (m-Ins), normal creatine (Cr), high levels of choline (Cho), and reduced N-acetylaspartate (NAA) (3). In general, MR spectroscopic studies of tumors show an increase in the Cho/Cr ratio and a decrease in the NAA/Cr ratio, with increasing histologic grade of malignancy and elevation of the Cho level regardless of the type of tumor (4–6). We present the case of a patient with a histologically proved low grade diffuse astrocytoma with mixed oligodendroglial components for whom multi-volume MR spectroscopy showed no elevation of Cho and the presence of high levels of m-Ins/glycine (Gly).
Case Report
A 33-year-old man presented with a 1-year history of progressively worsening headache. Immediately before admission, the patient experienced severe headaches associated with nausea and vomiting, acute confusion, and agitation. Unenhanced CT of the brain revealed a right frontotemporal mass. MR imaging of the brain confirmed the presence of an ill-defined hyperintense mass on T2-weighted and fluid-attenuated inversion recovery images involving the right insular cortex, with extension into the subcortical white matter and cortex and into the right frontal lobe (Fig 1). The lesion showed no contrast enhancement. To examine the entire tumor, six sections (grid thickness, 15 mm) of multi-voxel MR spectroscopic images were obtained (at 1.5 T). Each grid contained 40 individual voxels (individual voxels measured 1 × 1 × 1.5 mL). The study was conducted by using 1500/30, 135 (TR/first TE, second TE) and one signal intensity average (total acquisition time per section, 2 .56 min [Figs 2 and 3]). Manual baseline correction of all voxels of interest was performed. Areas under the curves for Cho, Cr, and NAA were obtained in 17 voxels containing only tumor (high T2 signal intensity and in the region of the biopsy) and in 19 voxels containing normal tissues (normal signal intensity). The peak areas (TE 135) in the voxels containing abnormal fluid-attenuated inversion recovery images signal intensity (and thus presumed to be tumors) were NAA (1.08 ± 0.28), Cr (1.23 ± 0.23), and Cho (0.90 ± 0.21). In the normal fluid-attenuated inversion recovery imaging signal intensity areas, the peak areas were NAA (1.83 ± 0.20), Cr (0.71 ± 0.17), and Cho (0.82 ± 0.21). Throughout the region of T2-weighted increased signal intensity, MR spectroscopy revealed a high m-Ins (and Gly) peak (3.5–3.7 ppm). Craniotomy with subtotal resection of the tumor was performed. A neuropathologic examination showed a well-differentiated fibrillary astrocytoma (World Health Organization grade II), with microcyst formation. Gemistocytic neoplastic cells were also identified. A prominent oligodendroglial neoplastic component was reflected in a population of round, regular nuclei cells surrounded by a clear perinuclear halo and the presence of pronounced perineuronal satellitosis.
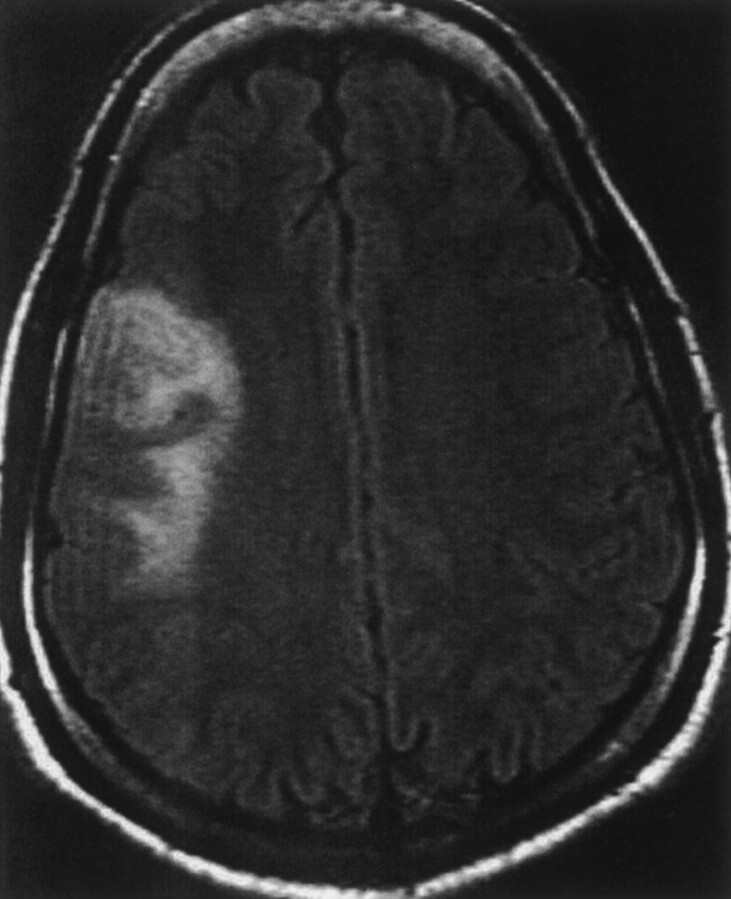
Fig 1.
Axial view fluid-attenuated inversion recovery image shows increased signal intensity involving cortex and white matter in the right insular and frontal regions.
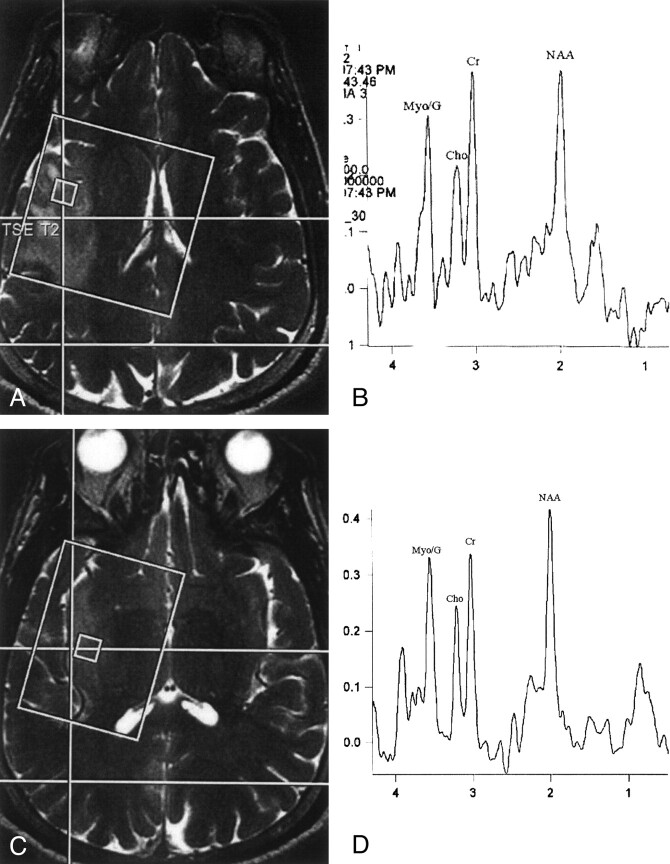
Fig 2.
A, Localizer T2-weighted MR image shows position of voxel within the area of high signal intensity in the right insula.
B, Proton MR spectroscopic image (TE 30) obtained from the image shown in A shows low NAA, elevated Cr and m-Ins/Gly (MyoG), and normal appearing Cho.
C, Localizer T2-weighted MR image, obtained 15 mm below the level at which the image shown in A was obtained, shows position of the voxel within area of increased signal intensity in the right insula.
D, Proton MR spectroscopic image (TE 30) obtained from the image shown in C shows mildly decreased NAA, normal Cho to Cr relationship, and elevated m-Ins/Gly. The spectra shown are similar and are representative of the pattern seen throughout the lesion.
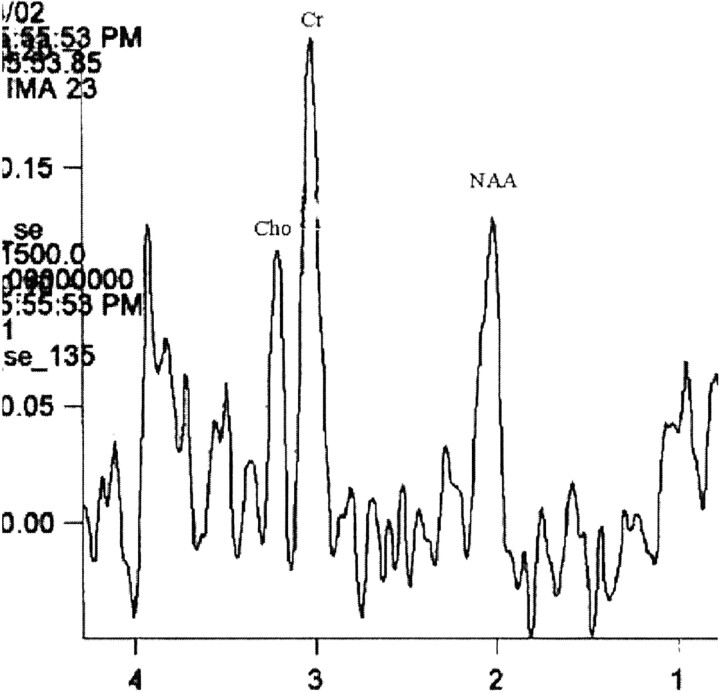
Fig 3.
Proton MR spectroscopic image (TE 135) obtained from a voxel within the area of T2 signal intensity abnormality. This long TE study confirms that the low levels of Cho and NAA revealed by short TE MR spectroscopy are not due to relaxation effects because they continue to be low. The Cr peak is high.
Discussion
In vitro studies of cultured major neural cell lines shed light regarding the metabolic patterns that can be discriminated by MR spectroscopy (7). In vitro, purified normal rodent oligodendrocytes and their precursors have higher levels of Gly than those levels found in other types of CNS cells (7). In addition, in vitro studies of human cells have shown that oligodendrocyte type 2 astrocyte progenitor cells and oligodendrocytes contain high levels of Gly, contrary to neurons and type 1 astrocytes that contain low levels of inositols and no Gly (7). When the MR spectroscopic imaging results of multiple sclerosis plaques are correlated with their histology, higher levels of m-Ins are found in regions containing predominantly fibrillary gliosis than in those with preferential protoplasmic gliosis (8). m-Ins is a sugar-like molecule found in astrocytes and is considered the most important osmolyte and cell volume regulator (9). m-Ins is also considered to be an astrocyte marker (9). The m-Ins peak as shown by MR spectroscopy also contains a contribution from Gly. Gly is the simplest nonessential amino acid abundant in mammalian fluids and tissues (10). Gly functions in the mammalian brain as a neurotransmitter and/or neuromodulator (10). Two types of Gly transporters (GlyT2 and GlyT1) have been described. GlyT2 is related to glycinergic neurons and GlyT1 is expressed by astrocytes involved in extracellular Gly regulation (10).
One study showed an increase in the m-Ins levels with decreasing histologic grade of malignancy (3). Gutowski et al (11) reported a histopathologically confirmed case of oligodendroglial gliomatosis cerebri with high levels of Gly/inositol revealed by MR spectroscopy, which they explained by the cell lineage of the tumor. In an article describing the MR spectroscopic imaging results in two cases of bilateral thalamic gliomas, the Cho levels were not significantly elevated, the Cr levels were high, and the NAA was only mildly reduced (12). This pattern is similar to that found in our case. The authors thought that the low grade of the tumors explained the lack of Cho elevation and offered no explanation for the increased Cr level. The level of the inositols and Gly were not assessed because their MR spectroscopic studies used only long TE.
In our patient, the peak found at 3.5 to 3.7 ppm may be explained by a high concentration of m-Ins reflecting the low histologic grade of the tumor with a contribution from Gly due to the presence of oligodendroglial cells. An elevation of Cho shown by MR spectroscopy is generally correlated with cellular proliferation. In our patient, elevation of the Cho peak was not qualitatively obvious but the areas under the curves showed that in the regions of tumor, the levels of this metabolite were minimally increased. We do not think that this increase in Cho was significant. The lack of significant elevation of the Cho peak is difficult to explain but may be accounted for by a lesion with a low growth fraction (ie, relatively fewer numbers of neoplastic cells in the active division phase of the cell cycle) (13). In addition, gemistocytes in a nonproliferative state (G0 phase of the cell cycle) may have contributed to the low Cho level. The normal cell cycle consists of an orderly sequence of phases (G1, S, G2, M, G0) and external and internal regulatory signals that stimulate or inhibit cell growth with checkpoints that guarantee that two identical daughter cells will be produced (13). In cancer cells, checkpoints are not rigorous, allowing production of cells with genetic changes (13). During the various phases of the cell cycle, synthesis, turnover, degradation, and accumulation of phospholipids (principally phosphatidylcholine) are taking place in preparation for cell division (14). Phosphatidylcholine constitutes approximately 40% of the cell membrane (6). Hydrolysis of phosphatidylcholine produces phosphocholine and phosphoethanolamine (14). Proton MR spectroscopy allows detection of phospholipid metabolites under the Cho peak at 3.2 ppm, which is composed of the signals from Cho-containing compounds such as glycerophosphocholine, phosphocholine, and free Cho (14). Short TE MR spectroscopy is needed to visualize m-Ins and Gly, and in this sequence, the Cho peak may have been relatively low because of relaxation effects. In our case, we also obtained MR spectroscopic images with long TE (TE 135), which also showed no elevation of the Cho peak, thus confirming the true nature of this finding. Additionally, in our case, the NAA peak was only minimally decreased and only in some of the voxels. NAA is a very sensitive marker of neuronal replacement, destruction, and/or dysfunction. That lipids and lactate were not observed is in accordance with the histologic low grade of the mass.
Histologic distinction between diffuse astrocytoma, mixed astrocytoma/oligodendroglioma, and oligodendroglioma is variable among observers, especially when the tumor shows areas of heterogeneous glial differentiation (15). Within oligodendrogliomas, gemistocytes are glial fibrillary acidic protein-positive cells (16). The presence of a preponderant gemistocytic component in astrocytomas is considered to be a poor prognostic sign because it can be associated with P53 mutation and rapidly progressive disease (17, 18). Additionally, gemistocytes may produce cytokines and growth factors that may promote tumor growth (19). It is thus possible that the prognosis for our patient will be less favorable than that suggested by the histologic and MR spectroscopic imaging patterns.
In summary, this case illustrates that the MR spectroscopic imaging findings of a tumor can be subtle, as reflected by the relatively normal levels of Cho and isolated high levels of m-Ins/Gly. Variations in the Cho levels may be closely related to the low proportion of dividing cells in the tumor, disrupting the balance between rates of biosynthesis and degradation of membranes. In our case, this unusual MR spectroscopic imaging pattern occurred despite the presence of MR imaging features that suggested a tumor. We suggest that near normal appearing MR spectra should not dissuade one from considering the possibility of tumor if the MR imaging features of a lesion suggest such a pathologic abnormality.
References
- 1.Piepmier JM, Fried I, Makuch R. Low-grade astrocytomas may arise from different astrocyte lineage. Neurosurgery 1993;33:627–632 [DOI] [PubMed] [Google Scholar]
- 2.Miller R, Raff M. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci 1984;4:585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castillo M, Smith JK, Kwock L. Correlation of myo-inositol levels and grading of cerebral astrocytomas. AJNR Am J Neuroradiol 2000;21:1645–1649 [PMC free article] [PubMed] [Google Scholar]
- 4.Tamiya T, Kinoshita K, Ono Y, Matsumoto K, Furuta T, Ohmoto T. Proton magnetic resonance spectroscopy reflects cellular proliferative activity in astrocytomas. Neuroradiology 2000;42:333–338 [DOI] [PubMed] [Google Scholar]
- 5.Tedeschi G, Lundbom N, Raman R, et al. Increased choline signal coinciding with malignant degeneration of cerebral gliomas: a serial proton magnetic resonance spectroscopy imaging study. J Neurosurg 1997;87:516–524 [DOI] [PubMed] [Google Scholar]
- 6.Miller BL, Chang L, Booth R, et al. In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life Sci 1996;58:1929–1935 [DOI] [PubMed] [Google Scholar]
- 7.Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 1993;13:981–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bitsch A, Bruhn H, Vougioukas V, et al. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MRS spectroscopy. AJNR Am J Neuroradiol 1999;20:1619–1627 [PMC free article] [PubMed] [Google Scholar]
- 9.Danielsen ER, Ross B. Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases. New York: Marcel Decker;1999. :34
- 10.Verleysdonk S, Martin H, Willker W, Leibfritz D, Hamprecht B. Rapid uptake and degradation of glycine by astroglial cells in culture: synthesis and release of serine and lactate. Glia 1999;27:239–248 [DOI] [PubMed] [Google Scholar]
- 11.Gutowski NJ, Gomez-Anson B, Torpey N, Revesz T, Miller D, Rudge P. Oligodendroglial gliomatosis cerebri: (1)H-MRS suggests elevated glycine/inositol levels. Neuroradiology 1999;41:650–653 [DOI] [PubMed] [Google Scholar]
- 12.Esteve F, Grand S, Rubin C, et al. MR spectroscopy of bilateral thalamic gliomas. AJNR Am J Neuroradiol 1999;20:876–881 [PMC free article] [PubMed] [Google Scholar]
- 13.Schabel FM. The use of tumor growth kinetics in planning “curative” chemotherapy of advanced solid tumors. Cancer Res 1969;29:2384–2389 [PubMed] [Google Scholar]
- 14.Dirks PB, Rutka JT. Currents concepts in neuro-oncology: the cell cycle. Neurosurgery 1997;40:1000–1015 [DOI] [PubMed] [Google Scholar]
- 15.Podo F. Tumour phospholipids metabolism review article. NMR Biomed 1999;12:413–439 [DOI] [PubMed] [Google Scholar]
- 16.Watanabe T, Nakamura M, Kros JM, et al. Phenotypes versus genotype correlation in oligodendrogliomas and low grade diffuse astrocytomas. Acta Neuropathol (Berl) 2002;103:2671–2675 [DOI] [PubMed] [Google Scholar]
- 17.Krouwer HG, David RL, Silver P, Prados M. Gemistocytic astrocytomas: a reappraisal. J Neurosurg 1991;74:399–406 [DOI] [PubMed] [Google Scholar]
- 18.Watanabe K, Tachibana O, Yonekawa Y. Role of gemistocytes in astrocytoma progression. Lab Invest 1997;76:277–284 [PubMed] [Google Scholar]
- 19.Paulus W, Grothe C, Sensenbrenner M, et al. Localization of basic fibroblast growth factor: a mitogen and angiogenic factor in human brain tumors. Acta Neuropathol (Berl) 1990;79:418–423 [DOI] [PubMed] [Google Scholar]