Abstract
BACKGROUND AND PURPOSE: Dysembryoplastic neuroepithelial tumors (DNTs) are benign lesions affecting children and are associated with epilepsy. The goal of our study was to better characterize the clinical-radiologic-pathologic spectrum of DNTs (complex and simple forms only) in a series of 14 children.
METHODS: Clinical, neuroradiologic, and pathologic features of all cases were retrospectively studied.
RESULTS: Eleven cases of complex and three cases of simple DNTs were identified. Mean follow-up was 87 months, and no recurrence was recorded except for one case of simple DNT. We found that some neuroradiologic features may be helpful to support the diagnosis of DNT: presence of “septations,” triangular pattern of distribution, and absence of contrast enhancement.
CONCLUSION: The evidence of the specific glioneuronal element is found by pathologic examination, but the typical neuroradiologic aspect of DNT suggests this diagnosis preoperatively. Radiologic examination may be helpful for the diagnosis of DNT when pathologic findings are inconclusive.
First described in 1988 by C. Daumas-Duport (1), dysembryoplastic neuroepithelial tumors (DNTs) are benign lesions affecting young people and are clinically characterized by drug-resistant partial seizures and normal neurologic examination. Neuroimaging typically shows a predominantly cortical and well-demarcated lesion (1, 2). Most DNTs do not display contrast enhancement (1, 3–6). Three histologic forms have been described (3, 7). The complex form is characterized by the association of a specific glioneuronal element (SGNE) with glial nodules and a multinodular architecture. Foci of cortical dysplasia are common. The simple form demonstrates only the SGNE (7). A third, “nonspecific,” form of DNT does not show the SGNE but displays the same clinical and neuroimaging features as complex DNT (3). On pathologic examination, this form may mimic any kind of glioma and, consequently, its existence is debated. Daumas-Duport et al define the clinical-radiologic criteria of DNT as follows: 1) partial seizures, with or without secondary generalization, beginning before the age of 20 years, 2) no neurologic deficit or stable congenital deficit, 3) cortical location of the lesion as best demonstrated by MR imaging, and 4) neither mass effect nor peritumoral edema findings at imaging (3).
We report a series of 14 DNTs (with SGNE) in children. Clinical, neuroradiologic, and pathologic results were reviewed, and we found that additional neuroradiologic findings were helpful to perform the diagnosis of DNT. To date, all children are alive, in keeping with the benign clinical course of DNT.
Methods
Case Selection and Clinical Findings
Between 1986 and 2000, 14 typical DNTs were diagnosed in children at our institution on the basis of pathologic findings. All patients fulfilled the clinical-radiologic criteria defined by Daumas-Duport et al. Age at diagnosis, sex, symptom duration before surgical excision, and clinical presentation, including the type of epilepsy, were recorded. Type of resection (tumorectomy vs lobectomy) and extent of resection were defined on the basis of both postoperative reports and early postoperative imaging. At the time of the last medical examination or on the basis of information obtained from a questionnaire sent to the patients’ medical doctors, the following parameters were recorded: anticonvulsive treatment (yes-no), seizure frequency, and recurrence (yes-no).
Neuroimaging
Preoperative and postoperative neuroradiologic examinations were reviewed by a neuroradiologist (N.G.). A preoperative CT scan was available in eight cases, and an iodinated contrast media injection was done in six cases. Preoperative MR imaging findings were available in all cases, with gadolinium injection in 10 cases. Lesion location, white matter involvement, calcifications, cystic component, overlying skull deformation, contrast enhancement, lesion shape, and presence of “septations” were studied.
Pathologic Analysis
Surgical specimens were fixed in formalin or Bouin’s fixative and paraffin embedded. In each case, all available microscopic slides were reviewed by two pathologists (D.F.B. and C.F.). Glial cell type, the presence of anaplasia criteria (including atypias, mitosis, necrosis, and endothelial proliferation), dysplastic neurons, perivascular inflammation, white matter, and meningeal involvement were studied.
Results
Clinical Features
The age at surgery ranged from 3 to 18 years (mean, 10.1 years). Nine male and five female patients were included in the study. Partial seizures occurred in all cases and were subsequently generalized in seven cases (cases 3, 5, 6, 8, 10, 12, and 14). Seizures were drug-resistant in nine cases. Seizure duration ranged from 2 weeks to 14 years, with a mean duration of 36 months before surgery. Thirteen patients had no neurologic deficit, and one patient (case 8) had a congenital unilateral sensorimotor deficit that remained unchanged for 4 years. Three patients presented with behavior disorders (cases 2, 5, and 8) and two with mental retardation (cases 8 and 9). Total surgical excision (tumorectomy [cases 1–5 and 7–14] or lobectomy [case 6]) was performed and no adjuvant therapy was administered (Table 1).
TABLE 1:
Clinical findings
| Patient (no.) | Age (y)/Sex | Symptom Duration | Seizure Type | Extent of Resection | Type of Resection | Seizure Frequency at Last Medical Examination/Anticonvulsive Treatment | Tumor Recurrence | Follow-up (Months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 11/M | 7 months | Partial* | Total | Tumorectomy | 0/No | No | 91 |
| 2 | 15/M | 4 years | Partial* | Total | Tumorectomy | 0/No | No | 148 |
| 3 | 7/M | 9 months | Partial* | Total | Tumorectomy | 0/No | No | 165 |
| 4 | 14/M | 6 months | Partial | Total | Tumorectomy | 0/No | No | 29 |
| 5 | 9/F | 8 months | Partial | Total | Tumorectomy | 0/Yes | No | 32 |
| 6 | 9/M | 2 years | Partial* | Total | Lobectomy | 0/No | No | 63 |
| 7 | 12/F | 9 months | Partial | Total | Tumorectomy | 0/No | No | 25 |
| 8 | 4/F | 3 years | Partial* | Total | Tumorectomy | 0/No | No | 99 |
| 9 | 11/F | 7 years | Partial* | Total | Tumorectomy | 0/No | No | 87 |
| 10 | 8/M | 4 months | Partial | Total | Tumorectomy | Unchanged/yes | No | 36 |
| 11 | 3/M | 5 months | Partial* | Total | Tumorectomy | 0/Yes | No | 36 |
| 12 | 18/F | 14 years | Partial* | Total | Tumorectomy | 0/No | No | 167 |
| 13 | 6/M | 2 years | Partial* | Total | Tumorectomy | 0 then recurrence/yes | Yes | 125 |
| 14 | 14/M | 2 weeks | Partial | Total | Tumorectomy | 0/No | No | 117 |
Drug-resistant seizures.
Neuroimaging
All cases displayed a cortical tumoral lesion that demonstrated neither perilesional edema nor mass effect on midline structures (Table 2, Figs 1A–D and 2A–D). Temporal, frontal, parietal, and occipital lobes were involved in six, seven, one, and one cases, respectively. One case (case 8) involved two adjacent lobes. On CT scans, the lesion appeared hypoattenuated in seven of eight available cases and heterogeneous in one case. No case demonstrated calcification, and two cases showed small cysts (cases 7 and 8). On T1-weighted images (Fig 1A), the lesion appeared to be hypointense in 13 cases and heterogeneous in one case (case 7). On T2-weighted images (Fig 1B), the lesion was of high signal intensity in all cases. A deformation of the overlying skull was observed in six cases. Gadolinium injection induced a tumor enhancement in three of 10 available cases, always with a nodular pattern. One of these cases had major hemorrhagic changes (case 7; Fig 2). In all cases, the lesion exceeded the thickness of the normal cortex and involved the white matter. In eight cases, the tumor width was maximal at the cortical level and decreased toward the ventricles, leading to a triangular pattern (Fig 1D) of distribution usually best seen on coronal images. The tumor boundaries were also rectilinear in three other cases but with a rectangular pattern (Fig 1C) of distribution, whereas the three remaining lesions were rounded. In all but two cases (cases 9 and 11), the lesion seemed to be divided by thin septa (Fig 1A–C). These septa were best seen on high-resolution MR images (inversion recovery 3-mm-thick sections, 3D T1-weighted images of 1-mm-thick sections) and had the same intensity as normal cortex. We named them “septations.” Consequently, most lesions were well-demarcated triangular lesions (57% of cases), did not display enhancement after contrast material injection (78% of cases), and showed septations (85% of cases). The association of these three features was observed in five (36%) of 14 cases.
TABLE 2:
Neuroradiologic findings
| Patient (no.) | Location | CT |
MR |
Distribution | Septations | Skull Erosion | ||
|---|---|---|---|---|---|---|---|---|
| Attenuation | Contrast | T1/T2 | Contrast | |||||
| 1 | Frontal | Hypoattenuated | NA | ↓/↑ | − | Rectangular | + | + |
| 2 | Temporal | NA | NA | ↓/↑ | − | Triangular | + | − |
| 3 | Temporal | Hypoattenuated | − | ↓/↑ | NA | Triangular | + | − |
| 4 | Frontal | Hypoattenuated | − | ↓/↑ | − | Rectangular | + | + |
| 5 | Temporal | NA | NA | ↓/↑ | Nodular | Round | + | + |
| 6 | Temporal | Hypoattenuated | − | ↓/↑ | NA | Triangular | + | + |
| 7 | Occipital | Heterogeneous | Nodular | H/↑ | Nodular | Triangular | + | − |
| 8 | Frontoparietal | NA | NA | ↓/↑ | Nodular | Rectangular | + | − |
| 9 | Temporal | NA | NA | ↓/↑ | − | Triangular | − | − |
| 10 | Frontal | Hypoattenuated | − | ↓/↑ | NA | Triangular | + | + |
| 11 | Temporal | NA | NA | ↓/↑ | − | Triangular | − | − |
| 12 | Frontal | NA | NA | ↓/↑ | − | Triangular | + | − |
| 13 | Frontal | Hypoattenuated | − | ↓/↑ | NA | Round | + | + |
| 14 | Frontal | Hypoattenuated | − | ↓/↑ | − | Round | + | − |
Note.—NA, not available; MRI, T1/T2 = T1-weighted image/T2-weighted image; ↓, hypointense lesion; ↑, hyperintense lesion; H, heterogeneous lesion; −, negative; +, positive.
Fig 1.
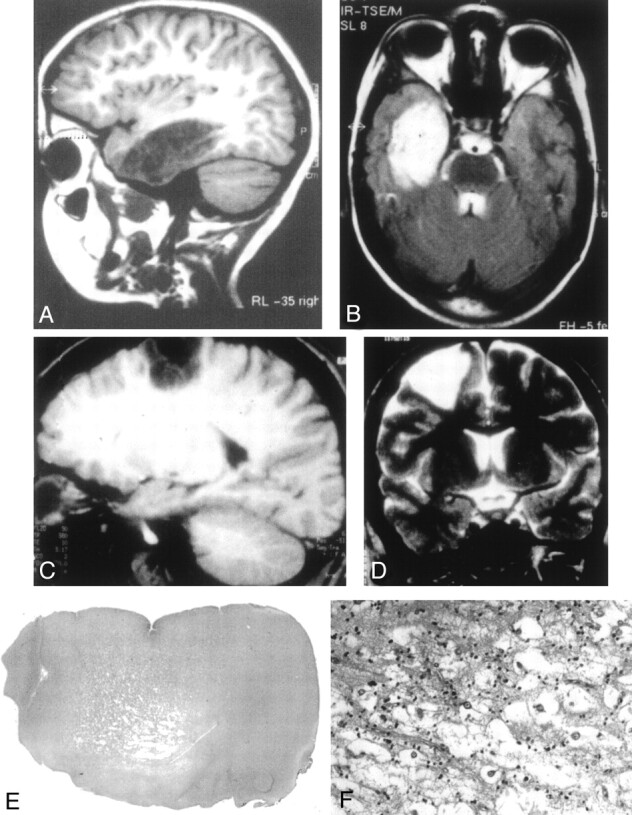
Typical DNT findings.
A, Sagittal T1-weighted MR image shows a large lesion of low signal intensity involving the temporal lobe, without edema or mass effect and corresponding to a complex form of DNT. The lesion is divided by septations leading to an alveolar aspect.
B, The lesion is of high signal intensity on this T2-weighted MR image. The septations appear to be of low signal intensity.
C, Sagittal T1-weighted MR image shows a frontoparietal DNT with sharp boundaries and a rectangular pattern of distribution.
D, Coronal T2-weighted MR image illustrates the triangular pattern of distribution typical of DNT, with a tumor width that is maximal at the cortical level and decreases toward brain ventricles.
E, Low-magnification view showing the cortical location and the nodular architecture typical of DNT (hematoxylin phloxin-saffron, magnification ×10).
F, The glio-neuronal specific element is composed of oligodendrocyte-like cells surrounding areas of mucoid substance containing “floating neurons” (hematoxylin phloxin-saffron, magnification ×300).
Fig 2.
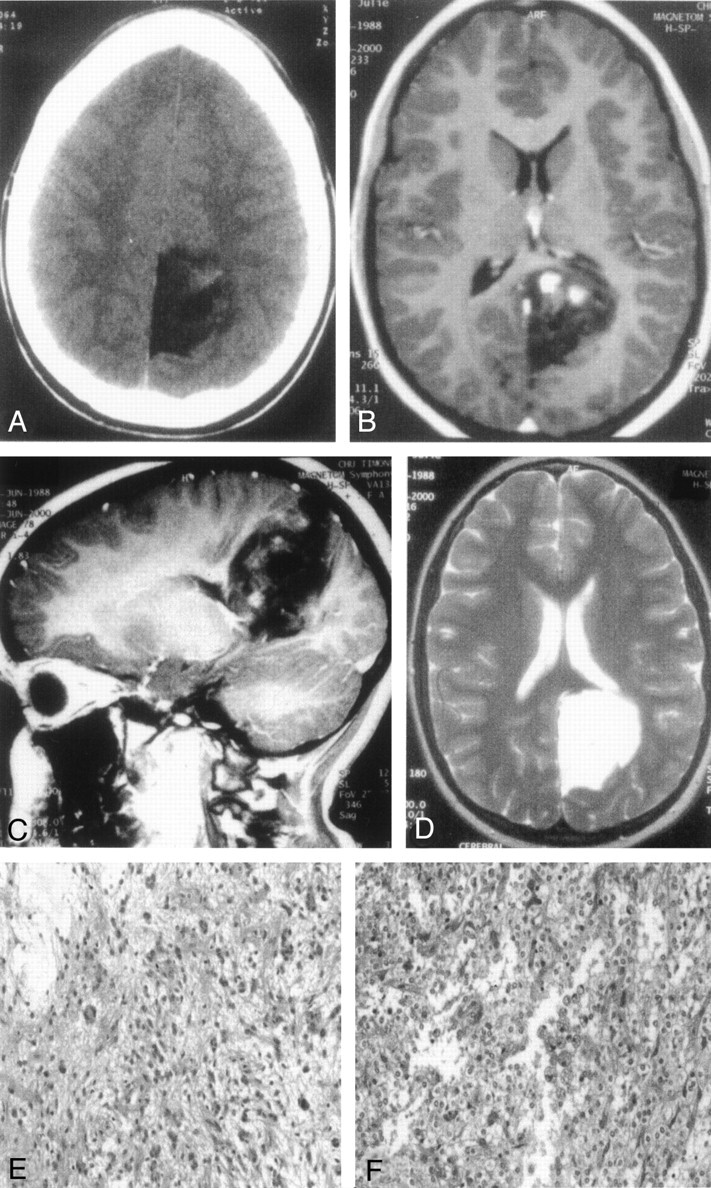
DNT involving the occipital lobe and presenting hemorrhagic changes (case 7).
A, On CT scan, the lesion appears of low attenuation and shows a nodular enhancement after contrast injection.
B and C, On transverse (B) and sagittal (C) T1-weighted MR images, the DNT is of low signal intensity, displays septations, but shows three areas of hyperintensity after gadolinium injection, mimicking a glioma.
D, Transverse T2-weighted MR image shows the absence of edema and mass effect on median structure.
E, Marked nuclear atypias can be observed in the glial areas of DNT (hematoxylin phloxin-saffron, magnification ×200).
F, An oligodendroglioma-like area showing major hemorrhagic changes characterized by numerous hemosiderin-laden histiocytes (hematoxylin phloxin-saffron, magnification ×200).
Histologic Findings
A typical specific SGNE was clearly identifiable in all cases (Fig 1E and F). In three cases (cases 12, 13, and 14), the SGNE represented the whole lesion, leading to the diagnosis of the simple form of DNT (Table 3). The 11 other cases displayed glial nodules and were classified as complex DNTs. In these glial nodules, oligodendrocyte-like areas were observed in all cases, associated with piloid cells in two cases and fibrillary astrocytes in one case. Calcifications were detected in four cases. Nuclear pleiomorphism (Fig 2E) was noted in three complex DNTs, including one case with strong atypias (case 2). Rare mitoses were observed in one case (case 2). One case demonstrated scarce endothelial proliferation (case 9). Necrosis was not recorded. Discrete and focal perivascular lymphocytic cuffing was present in three cases (cases 6, 7, and 8). One of them (case 7) presented major hemorrhagic changes (Fig 2F). In six cases, the lesion involved the leptomeninges, and the SGNE was occasionally observed in this location. Numerous neurons were entrapped in the glial component in all cases, but no dysplasic neurons typical of gangliogliomas were observed. Cortical dysplasia was observed in seven of eight complex DNTs where enough cortex was available at pathologic examination.
TABLE 3:
Pathologic findings
| Patient | Cortical Dysplasia | Glial Nodule Cell Type | Calcifications | Atypias | Endothelial Proliferation | Perivascular Inflammation | Meningeal Involvement | |
|---|---|---|---|---|---|---|---|---|
| 1 | C.U. | + | O | − | − | − | − | + |
| 2 | M.I. | NA | O + P | + | ++ | − | − | + |
| 3 | R.E. | + | O | + | − | − | − | − |
| 4 | S.C. | + | O | − | − | − | − | + |
| 5 | D.E. | NA | O | + | − | − | − | − |
| 6 | B.E. | + | O + A | − | − | − | + | − |
| 7 | B.O. | − | O + P | − | + | − | + | + |
| 8 | L.A. | + | O | − | − | − | + | + |
| 9 | Z.E. | + | O | + | + | + | − | + |
| 10 | A.N. | NA | O | − | − | − | − | − |
| 11 | C.H. | + | O | − | − | − | − | − |
| 12 | A.Y. | + | None | − | − | − | − | − |
| 13 | P.I. | NA | None | − | − | − | − | − |
| 14 | C.E. | NA | None | − | − | − | − | − |
Note.—O, oligodendrocytes; P, piloid cells; A, fibrillary astrocytes; −, negative; +, positive; NA, not available.
Follow-up
The follow-up duration ranged from 25 to 167 months, with a mean of 87 months. At the time of the last examinations, 12 patients were free of seizures, and only one still used an anticonvulsive treatment. Seizure frequency was unchanged in one case (case 10). In the remaining case (case 13), seizures began again after a seizure-free period of 2 years, and tumor recurrence was observed on MR images 125 months after the initial excision. The child underwent surgery at another center, and we could not study the new pathologic specimen. No neuroradiologic evidence of recurrence was observed in the 13 others cases.
Discussion
Clinical presentation of DNT is stereotyped: a long history of partial drug-resistant seizures and the absence of progressive neurologic deficit (1, 3–6). DNTs are stable and benign lesions, although one case of malignant transformation 11 years after initial excision has been reported (8). Currently, lesion stability cannot often be evaluated, because the occurrence of partial seizures leads to neuroimaging and subsequent surgical excision. Surgical excision remains necessary, because it provides the best chance to cure epilepsy and prevents hemorrhagic complications (9). Regarding our patients, most lesions were initially considered as tumors on the basis of neuroimaging findings, so a simple tumorectomy was performed in 13 cases, whereas only one patient underwent classic epilepsy surgery. All but two patients were seizure free after excision, so it seems that tumorectomy is sufficient to cure epilepsy in most cases. The single case of recurrence (case 12) was initially a simple form of DNT. It is currently the only reported case of recurrence except for a case of malignant transformation (8).
Typically, DNTs display hypointensities on T1-weighted MR images and hyperintensities on T2-weighted MR images (3, 4, 10). Edema and mass effect on midline structures are lacking (3, 4, 10), although they may be observed in case of hemorrhagic complications (9, 11). Diffuse astrocytomas and World Health Organization (WHO) grade II oligodendrogliomas may in some cases share this neuroradiologic aspect (12). Nevertheless, it is of the utmost importance to distinguish DNTs from gliomas, because DNTs can be cured by surgery alone. This is of particular interest in children because of the highly deleterious effect of adjuvant therapies. Generally, pathologic examination leads to the diagnosis of DNT if the SGNE is present; however, it appears obvious that this diagnosis must be affirmed cautiously, because some oligodendrogliomas harboring cystic changes and entrapping neurons may mimic the SGNE (13). Moreover, the aggressiveness criteria, including mitosis, ischemic necrosis, capillary proliferation, nuclear atypias, and meningeal involvement are usually observed in malignant tumors but do not exclude the diagnosis of DNT (1, 3, 5, 6, 14, 15). For all these reasons, the diagnosis of DNT must be the result of a multidisciplinary discussion including clinicians, neuroradiologists, and pathologists.
With regard to our series, neuroimaging findings seem to be very helpful in the diagnosis of DNT. In addition to lack of peritumoral edema and mass effect on midline structures, triangular pattern of distribution and presence of septations are frequently observed. Because DNTs are thought to be of dysembryoplastic origin, the triangular pattern of distribution may be related to the radial glial fibers pathway. Septations probably correspond to the lobular aspect described elsewhere (2). They are true septations and are not caused by distortions of sulci and reflection. They may reflect the nodular architecture of the DNT and the demarcations between normal cortex, densely cellular zones (glial nodules), and loose zones (SGNE). Therefore, it is likely that septations do not occur in the nonspecific form of DNT.
To compare neuroimaging findings of DNTs with those of different brain lesions, we searched for septations and triangular pattern of distribution in 16 other lesions in children, including eight oligodendrogliomas, four gangliogliomas, one xanthoastrocytoma, one tuber, one glioneuronal hamartoma, and one desmoplastic infantile ganglioglioma. On the whole, in our experience, the association of the two parameters “septations” and “triangular pattern” was observed in only one WHO grade II oligodendroglioma diagnosed on the basis of simple biopsy findings. Therefore, we cannot rule out that this biopsy sample was a glial nodule of a DNT. Among the four gangliogliomas, one showed septations and another was triangular. None of the remaining lesions demonstrated septations or triangular shape. Consequently, these two features strongly suggest the diagnosis of DNT and may be helpful in distinguishing them from gliomas on the basis of neuroimaging findings. The absence of contrast enhancement also helps guide the diagnosis. When present, contrast enhancement is nodular in most cases (1, present series), as opposed to gangliogliomas that often demonstrate diffuse contrast enhancement (16, present series). In our series, an underlying skull deformation was observed in only six of 14 cases, in part because in most cases the lesion was distant from bone; however, despite its presence in the case of slowly growing tumors, this element remains a good argument for DNT (1).
Conclusion
When a child presents with a cortical epileptogenic lesion that may be a DNT, clinical, neuroradiologic, and pathologic data must all be considered to achieve the accurate diagnosis, optimize the management of the patient, and avoid deleterious treatment. In the case of inappropriate pathologic sampling or diagnostic difficulties, some neuroradiologic features including a triangular pattern of distribution and the presence of septations may aid in the diagnosis.
Acknowledgments
We are grateful to Dr. Agnes, Dr. Brochet, Prof. Chabrol, Prof. Coubes, Dr. Donnet, Dr. Dravet, Dr. Gonzalez, Dr. Lamoureux, Prof. Mancini, Dr. Nobili, Dr. Pastor, Dr. Pebeyre, Dr. Pedespan, Dr. Perrouty, Dr. Piron, Dr. Pujibet, Dr. Saban, Dr. Tayleigne, Dr. Tran, Dr. Viallat, and Dr. Weck, for their assistance concerning patients follow-up. We thank Prof. Roche for critical reading of this manuscript.
Footnotes
Supported by the Association pour la Recherche contre le Cancer, the Groupement des Entreprises Françaises dans la Lutte contre le Cancer, and institutional grants to D.F.B.
References
- 1.Daumas-Duport C, Scheithauer BW, Chodkiewicz JP, et al. Dysembryoplastic neuroepithelial tumor: a surgically curable tumor of young patients with intractable partial seizures: report of thirty-nine cases. Neurosurgery 1988;23:545–556 [DOI] [PubMed] [Google Scholar]
- 2.Lee DY, Chung CK, Hwang YS, et al. Dysembryoplastic neuroepithelial tumor: radiological findings (including PET, SPECT, and MRS) and surgical strategy. J Neurooncol 1995;47:167–174 [DOI] [PubMed] [Google Scholar]
- 3.Daumas-Duport C, Varlet P, Bacha S, et al. Dysembryoplastic neuroepithelial tumors: nonspecific histological forms: a study of 40 cases. J Neurooncol 1999;41:267–280 [DOI] [PubMed] [Google Scholar]
- 4.Fomekong E, Baylac F, Moret C, et al. Dysembryoplastic neuroepithelial tumors: analysis of 16 cases. Neurochirurgie 1999;45:180–189 [PubMed] [Google Scholar]
- 5.Lemesle M, Borsotti JP, Justrabo E, et al. Dysembryoplastic neuroepithelial tumors: a benign tumor cause of partial epilepsy in young adults. Rev Neurol (Paris) 1996;152:451–457 [PubMed] [Google Scholar]
- 6.Raymond AA, Halpin SFS, Alsanjari N, et al. Dysembryoplastic neuroepithelial tumour: features in 16 patients. Brain 1994;117:461–475 [DOI] [PubMed] [Google Scholar]
- 7.Daumas-Duport C. Dysembryoplastic neuroepithelial tumours. Brain Pathol 1993;3:283–295 [DOI] [PubMed] [Google Scholar]
- 8.Hammond RR, Duggal N, Woulfe JM, Girvin JP. Malignant transformation of a dysembryoplastic neuroepithelial tumor: case report. J Neurosurg 2000;92:722–725 [DOI] [PubMed] [Google Scholar]
- 9.Thom M, Gomez-Anson B, Revesz T, et al. Spontaneous intralesional haemorrhage in dysembryoplastic neuroepithelial tumours: a series of five cases. J Neurol Neurosurg Psychiatry 1999;67:97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuroiwa T, Bergey GK, Rothman MI, et al. Radiologic appearance of the dysembryoplastic neuroepithelial tumor. Radiology 1995;197:233–238 [DOI] [PubMed] [Google Scholar]
- 11.Ostertun B, Wolf HK, Campos MG, et al. Dysembryoplastic neuroepithelial tumors: MR and CT evaluation. AJNR Am J Neuroradiol 1996;17:419–430 [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch JF, Sainte Rose C, Pierre-Kahn A, et al. Benign astrocytic and oligodendrocytic tumors of the cerebral hemispheres in children. J Neurosurg 1989;70:568–572 [DOI] [PubMed] [Google Scholar]
- 13.Leung SY, Gwi E, Ng HK, et al. Dysembryoplastic neuroepithelial tumor: a tumor with small neuronal cells resembling oligodendroglioma. Am J Surg Pathol 1994;18:604–614 [PubMed] [Google Scholar]
- 14.Guesmi H, Houtteville JP, Courthéoux P, et al. Dysembryoplastic neuroepithelial tumors: report of 8 cases including two with unusual localization. Neurochirurgie 1999;45:190–200 [PubMed] [Google Scholar]
- 15.Honavar M, Janota I, Polkey CE. Histological heterogeneity of dysembryoplastic neuroepithelial tumour: identification and differential diagnosis in a series of 74 cases. Histopathology 1999;34:342–356 [DOI] [PubMed] [Google Scholar]
- 16.Provenzale JM, Ali U, Barboriak DP, et al. Comparison of patient age with MR imaging features of gangliogliomas. AJR Am J Roentgenol 2000;174:859–862 [DOI] [PubMed] [Google Scholar]


