Abstract
BACKGROUND AND PURPOSE: The accuracy of cranial sonography (US) in characterizing white matter (WM) injury in the premature infant is unclear. This study was aimed to assess the sensitivity and specificity of serial cranial US during the first 6 weeks of life in comparison to MR imaging at term (week of expected delivery) in characterizing the presence of WM injury in a cohort of 96 very low birth weight (VLBW) infants.
METHOD: A blinded investigator reviewed serial cranial sonograms for the presence of WM echolucency and echodensity, including its duration and extent. These abnormalities were compared with a second independent investigator’s evaluation to determine the sensitivity and specificity of cranial WM abnormalities at US.
RESULTS: The presence of prolonged echodensity (>7 days) in the WM on neonatal cranial sonograms demonstrated low sensitivity (26%) and a low positive predictive value (36%) for the presence of noncystic WM injury, as detected on MR images at term. Extensive cystic lesions detected on MR images were all identified during earlier cranial US.
CONCLUSION: Neonatal cranial US of the VLBW infant demonstrates high reliability in the detection of cystic WM injury but has significant limitations in the demonstration of noncystic WM injury. This deficiency of neonatal cranial US is important, because noncystic WM injury is considerably more common than cystic WM injury.
Cranial sonography (US) is the most widely used neuroimaging procedure in the study of the premature infant. The technique is readily applied at the bedside, does not involve ionizing radiation, is cost-effective, and can be used sequentially. In recent years, the particular importance of cerebral white matter (WM) injury in the genesis of neurologic sequelae in the premature infant has been increasingly recognized (1). WM injury in the premature infant is characterized by focal necroses deep in the WM, with subsequent cyst formation, and the more common, diffuse and noncystic WM injury characterized by loss of early differentiating oligodendrocytes, astrogliosis, and subsequent impairment of myelination (1). Although cranial US has been shown to be reliable in the detection of focal areas of cystic WM injury (2–5), the value of this imaging technique in detecting the more common diffuse, noncystic WM injury is unclear. MR imaging has previously been compared with cranial US for the detection of cerebral injury in the premature infant (6–9). The investigators concluded that MR imaging was superior in the definition of the extent of WM injury and associated abnormalities, such as abnormal signal intensity in the posterior limb of the internal capsule. All of these studies, however, were retrospective and related the MR imaging and cranial US findings in relatively small, select clinical cohorts of premature infants with vastly mixed cerebral lesions. Thus, our prospective study included an unselected cohort of very low birth weight infants (VLBW). Our aim was to define the sensitivity and specificity of cranial WM abnormalities found during serial US studies in the first 6 weeks of life for the detection of both cystic and noncystic WM injury observable at term (week of expected due date) on MR images.
Methods
Subjects
From November 1998 to December 2000, 100 consecutively born, premature infants with VLBW, or a birth weight less than 1500 g, were recruited into a study of advanced imaging techniques for an assessment of brain injury in the premature infant. These infants represented 98% of eligible infants admitted to a regional level III neonatal intensive care unit. The mean gestational age of the infants was 27.9 weeks ± 2.4, with mean birth weight of 1063 g ± 292. The characteristics of these infants are summarized in Table 1.
TABLE 1:
Characteristics of the total cohort of VLBW
| Characteristic | No. of Infants (n = 96) |
|---|---|
| Birth weight | 1063 ± 292 |
| Gestational age, weeks | 27.9 ± 2.4 |
| Male/Female | 48/48 |
| Singletons | 64 |
| Intrauterine growth restriction | 25 |
| Prolonged Ruptured Membranes | 22 |
| Oxygen therapy, days | 26.8 ± 38 |
| Mean arterial pressure <30 mmHg | 18* |
| Inotropic use for low blood pressure | 34 |
| Patent ductus arteriosus† | 45 |
| Intraventricular hemorrhage grade III or IV | 4 |
| Pneumothorax | 6 |
Of 66 infants with intra-arterial catheter monitoring.
Confirmed at echocardiography.
Permission for this study was granted by the Canterbury Regional Health Research Ethics Committee, and written parental consent was obtained for each infant.
Cranial US
Sequential cranial US scans were obtained by using a standardized protocol, with images acquired at a minimum of the first 48 hours, at 5–7 days, and at 4 and 6 weeks of age. If any abnormality was detected, weekly US assessments were undertaken. Sonograms were acquired with 7.5- or 8.5-MHz probes by using various sonography machines (XP-10, Aspen, and Sequoia; Acuson, Mountain View, CA). A standardized series of images was obtained at every sonographic examination. Six coronal views (at the level of the orbits, middle cerebral arteries, third ventricle, fourth ventricle, bodies of lateral ventricles, and centrum semiovale), and five sagittal-parasagittal views (midline, ventricles, and paraventricular WM) were obtained via the anterior fontanelle. Three coronal images (occipital horns, splenium, and centrum semiovale) and three sagittal-parasagittal images (midline, trigone, and occipital horns) were obtained via the posterior fontanelle. WM echogenicity in frontal regions was assessed on the anterior fontanelle sonograms. In parieto-occipital regions, the echogenicity was assessed from the anterior fontanelle approach and corroborated with the posterior fontanelle view.
Analysis of Cranial Sonograms
An experienced radiologist (N.J.A.) who was blinded to the infant’s clinical course and to the MR imaging results analyzed all cranial US scans. Blinded repeat readings were undertaken for both cranial US and MR imaging to determine the validity and reliability of the measures. Although the cranial US scans were analyzed extensively, for the purposes of this study, WM echodensities and echolucencies were emphasized. Abnormal periventricular echodensity was defined as abnormal signal intensity in the periventricular WM with signal intensity characteristics greater than those in the choroid plexus (10). Peritrigonal flush was excluded. Echodensity in the periventricular WM was classified as absent, transient (<7 days in duration), prolonged (>7 days n duration), or were described as echodensities evolving to echolucencies (cysts) (Fig 1). The extent of the WM echodensity or echolucency was recorded by the regions involved: frontal, parietal, or occipital. The number and size of the cystic lesions were classified as single or multiple and as small, moderate, or large (11).
Fig 1.
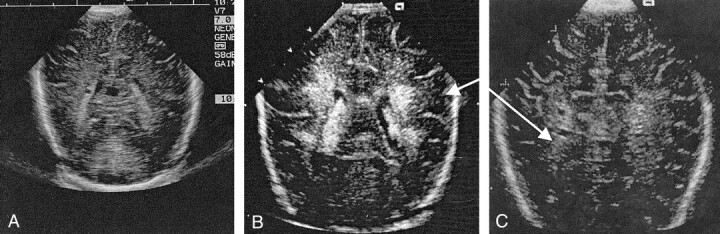
Representative cranial sonograms.
A, Normal image, coronal view.
B, Flaring in the periventricular WM, coronal image.
C, Cystic PVL with a cystic abnormality in a region of a signal intensity abnormality, axial view.
MR Imaging
MR imaging was performed with a 1.5-T system (Signa; GE Medical Systems, Milwaukee, WI). For the acquisition of the primary MR imaging data, two imaging modes were applied: a three-dimensional (3D) Fourier-transform spoiled gradient-recalled (SPGR) sequence (1.5-mm coronal sections; flip angle, 45°; TR/TE, 35/5; field of view, 18 cm; matrix, 256 × 256) and a double-echo (proton density [PD]–and T2-weighted) spin-echo sequence (3-mm axial sections; 3,000/36 and 162; field of view, 18 cm; matrix, 256 × 256, interleaved acquisition). An experienced neuroradiologist (S.W.) evaluated the MR images in a blinded manner for WM abnormality. Although the MR images were analyzed extensively, for the purposes of this study, we focused our definition of WM injury on the presence and severity of WM signal intensity abnormalities. The MR imaging diagnosis of WM signal intensity abnormality required T1 hyperintensity in the absence of marked T2 hypointensity in the periventricular WM; this was interpreted as astrogliosis (12). Areas of notable T1 hyperintensity and substantial T2 hypointensity were interpreted as signal intensity abnormalities suggestive of foci of hemorrhage. WM signal intensity was classified as normal, a focal signal intensity abnormality (eg, involving one lobar region only), an extensive signal intensity abnormality (eg, involving more than one lobar region), or a cystic change. We took the license of using a pathologic term cystic change for the MR imaging abnormality that is indicative of areas of tissue dissolution (characteristic of cystic periventricular leukomalacia [PVL]) Representative sagittal MR images in the infants with these grades of WM injury are shown in Figure 2.
Fig 2.
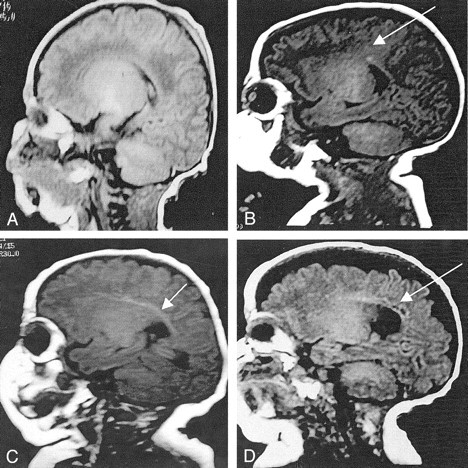
Representative sagittal T1-weighted MR images (3-mm sagittal T1; 550/12; FOV, 18 cm) in premature infants obtained at term.
A, Normal image.
B, Focal signal intensity abnormality (arrow).
C, Extensive signal intensity abnormality (arrow).
D, Cystic change (arrow).
Statistical Analysis
The value of cranial US as a predictor of WM abnormality, as shown on MR images at term, was assessed by calculating the sensitivity, specificity, positive predictive value, and negative predictive value of cranial US findings.
Results
Ninety-six infants completed the protocol of all cranial US scans during their admission. Four infants were excluded, because they were transferred to other units during their admission.
MR Imaging Findings
At term, 38 infants 40% had MR imaging findings indicative of WM injury (Fig 1B–D, Table 2). A total of 18 infants 19%) had extensive WM signal intensity abnormalities or cystic WM injury on MR images (Table 2)). Fourteen of these infants had extensive signal intensity abnormality (Fig 2C), usually with loss of WM volume and ventriculomegaly. The remaining four infants had cystic WM injury: two with bilateral cystic PVL, one with periventricular hemorrhagic infarction and a unilateral WM cyst, and one with severe posthemorrhagic ventriculomegaly and a single small periventricular cyst. The presence of cystic change in the WM is illustrated in Figure 2D.
TABLE 2:
Results of neonatal cranial US and MRI at term
| Cranial US Finding | No. of Infants with MRI WM Finding |
|||
|---|---|---|---|---|
| Normal (n = 58) | Focal Signal Abnormality (n = 20) | Extensive Signal Abnormality (n = 14) | Cystic Change (n = 4) | |
| Normal (n = 40) | 28 | 8 | 4 | 0 |
| Transient echodensity (n = 34) | 20 | 8 | 5 | 1 |
| Prolonged echodensity (n = 19) | 10 | 4 | 5 | 0 |
| Echolucencies (n = 3) | 0 | 0 | 0 | 3 |
Cranial US Findings
Forty infants 42% had persistently normal cranial US findings (Table 3). In 22 infants (23%), prolonged echodensities (>7 days) (n = 19) or echodensities evolved into echolucencies (cysts) in the periventricular WM (n = 3) (Table 3). The distribution of echodensities or echolucencies in the periventricular WM were found to be frontal-parietal-occipital (n = 4), frontoparietal (n = 1), parietal-occipital (n = 8), frontal (n = 2), parietal (n = 4), or isolated occipital (n = 3). The infants with echolucencies had a single encephalomalacic frontal cyst (secondary to apparent parenchymal hemorrhagic infarction, n = 1), multiple small and large parietal-occipital cysts (n = 2), or a single parietal cystic (n = 1).
TABLE 3:
Statistical comparison of neonatal cranial US and MRI findings at term
| Neonatal Cranial US Finding | MRI Finding at Term | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|
| No echodensity or transient echodensity < 7 days | Normal WM or focal signal intensity abnormality | 82% | 44% | 86% | 36% |
| Prolonged echodensity > 7 days | Extensive signal abnormality or cystic change | 26% | 85% | 36% | 82% |
| Echolucency | Cystic change | 75% | 100% | 100% | 98% |
Sensitivity, Specificity, and Predictive Values of Cranial US for WM Injury
Of the 74 infants with normal neonatal cranial US findings or only transient echodensity (<7 days) on serial cranial sonograms, at-term MR images in 10 (14%) showed evidence of notable WM injury, with an extensive signal intensity abnormality or cystic injury (Table 2). Of the 22 infants with more overt WM abnormalities on cranial sonograms, that is, prolonged echodensity (> 7days) or echolucency in the periventricular WM, 14 infants (64%) had normal or only focal signal intensity changes in the WM on MR images at term. The remaining eight infants with these WM abnormalities on cranial US scans had extensive or cystic WM injury on MR images at term (Table 2).
Of the 18 infants with extensive or cystic WM injury during MR imaging at term, only eight infants had prominent abnormalities (prolonged echodensity or echolucencies), detected with cranial US during their neonatal course, while 10 infants had either normal US findings or transient echodensity (<7 days) (Table 2). Neonatal cranial US results were abnormal in the four infants, with the most severe WM injury; findings included bilateral cystic WM injury in the two infants with severe PVL, cystic change with apparent periventricular hemorrhagic infarction in one infant, and severe posthemorrhagic ventriculomegaly in one infant. Cranial US did not depict the single minor cyst in the infant with severe posthemorrhagic ventriculomegaly, but it accurately depicted all other cystic lesions.
The sensitivity of the prominent neonatal cranial US abnormality of prolonged echodensity (>7 days) for overt WM injury on MR images at term, as manifested by extensive signal intensity abnormality or cystic change, was only 26% (Table 3). Moreover, the positive predictive value of this US finding was only 36%. For cystic changes on MR images at term, cranial US was more useful; that is, echolucency on US scans had a sensitivity of 75% and a specificity of 100% with high positive and negative predictive values (Table 3). No substantial alteration in these results occurred with an analysis of the extent of the cranial US abnormality. The sensitivity of normal cranial US findings or the finding of only transient echodensities for the finding of normal WM or only focal signal intensity abnormality on MR images at term was 82% (Table 3). However, the specificity was only 44%, and the negative predictive value was only 36%.
Discussion
To our knowledge, this study of 96 premature infants is the largest published prospective study of the accuracy of serial neonatal cranial US in the detection of cerebral WM injury, as identified with MR imaging at term. This study revealed several findings of clinical relevance. First, the findings show that cranial US has poor sensitivity for the detection of WM injury. In approximately 55% of infants with extensive signal intensity abnormalities or cystic changes in the cerebral WM, as shown with MR imaging at term, neonatal cranial US findings were either entirely normal or consisted of only transient echodensity. Thus, cranial US is not an adequate screening method for WM injury in the VLBW infant. Second, our findings showed that the cranial US abnormality of prolonged echodensity (>7 days) demonstrated a high false-positive rate with a low positive predictive value. Thus, only approximately 26% (five of 19) of infants with prolonged echodensity had an extensive signal intensity abnormality or cystic WM injury on MR images at term. In contrast to the poor sensitivity of the finding of prolonged echodensity in the prediction of WM injury, the cranial US finding of echolucency was sensitive for cystic WM injury. Nevertheless, based on MR imaging results at term, cystic WM injury occurred in only four of the 96 infants in this study, whereas extensive noncystic WM injury occurred in 14.
This study documents the low sensitivity of serial cranial US for the detection of WM injury in the premature infant during the first 6 weeks of life. These findings are entirely consistent with those of previous correlative neuropathologic studies demonstrating that as few as 30% of WM injuries, especially small areas of necrosis, diffuse gliosis, and myelin loss, are consistently detected in vivo with US (2–5). Our data are also consistent with those of previously published studies comparing cranial US and MR imaging in the premature infant. In a recent study of 32 VLBW infants, a sensitivity of 0.5 was found for a moderate or severe degree of echodensity with single cranial US (at days 1–95 of life) in the detection of simultaneous WM signal intensity abnormalities on MR images (6). In that study, a WM signal intensity abnormality was defined as diffuse, excessive, hyperintense area on T2-weighted MR images, a finding observed in nearly 80% of the infants at term equivalent. The frequency of this MR signal intensity abnormality at term equivalent was greater than at the earlier time points (25% in the first four postnatal days, 53% at postnatal days 7–95 but <37 weeks postconceptional age). Our study differs notably from this other investigation in that our hypothesis was defined a priori to examine the relationship of standard serial cranial US assessments in the first 6 weeks of life with MR imaging-defined WM injury at term, as manifested by more definable focal or extensive signal intensity abnormalities or cystic change (Fig 1). By term equivalent, the extent of WM injury is more clearly established with better delineation of cystic lesions and signal intensity abnormalities on both T1- and T2-weighted images, as well as the loss of WM volume with secondary ventriculomegaly. Moreover, in our study, we wished to address the accuracy of the finding of prolonged echodensity (>7 days) on serial cranial sonograms in the first 6 weeks of life, rather than the degree of echodensity on a single scan at a random time interval in the neonatal course, in predicting the presence and severity of WM injury.
The cranial US finding of prolonged echodensities demonstrated a low positive predictive value for the presence of extensive signal intensity abnormality in the cerebral WM at term. The detection of prolonged echodensities in the periventricular WM on cranial sonograms in the premature infant has been considered an important abnormal finding (10). However, echodensities in the posterior parietal region without any neuropathologic correlate (13) are common in the preterm infant. Our demonstration of the low positive predictive value of cranial US is also consistent with the findings of a recent study of 34 premature infants with a birth weight less than 1750 g (8). In this earlier report, WM findings from serial cranial US scans were compared with those of a single MR image obtained during the neonatal period (mean age at MR imaging, 18.7 days; mean gestational age, 33.2 weeks). In the 10 infants with prolonged WM echodensities (>7 days), only two had definite WM injury on neonatal MR images.
Conclusion
Our study of a large unselected cohort of VLBW infants shows that the finding of cranial US WM echodensities lacks sensitivity and positive predictive value for the presence of prominent WM injury, as defined by means of MR imaging at term. Our findings are consistent with related observations from neuropathologic studies and previous smaller published imaging series and emphasize the appreciable limitations of this bedside technology in the diagnosis of WM injury. Such limitations restrict the clinician’s ability to used cranial US to accurately diagnose this common cerebral disease in high-risk populations of premature infants.
Acknowledgments
We are grateful to the Neurologic Foundation of New Zealand, Health Research Council, and Lottery Health for their funding support; to the Canterbury District Health Board and the Christchurch Womens’ Health Service for their support; and to the Canterbury Radiology Group, Christchurch, New Zealand for their assistance with the acquisition of the MR images.
Footnotes
Supported by grants from the Neurological Foundation of New Zealand, Health Research Council of New Zealand and Lottery Health of New Zealand.
Presented at the Society for Pediatric Research, Baltimore, MD, May 2002.
References
- 1.Volpe JJ. Neurology of the Newborn. 4th ed. Philadelphia: WB Saunders,2001
- 2.Hope PL, Gould SJ, Howard S, Hamilton PA, Costello AM, Reynolds EO. Precision of ultrasound diagnosis of pathologically verified lesions in the brains of very preterm infants. Dev Med Child Neurol 1988;30:457–471 [DOI] [PubMed] [Google Scholar]
- 3.Paneth N, Rudelli R, Monte W, et al. White matter necrosis in very low birth weight infants: neuropathologic and ultrasonographic findings in infants surviving six days or longer. J Pediatr 1990;116:975–984 [DOI] [PubMed] [Google Scholar]
- 4.Carson SC, Hertzberg BS, Bowie JD, Burger PC. Value of sonography in the diagnosis of intracranial hemorrhage and periventricular leukomalacia: A postmortem study of 35 cases. Am J Neuroradiol 1990;155:595–601 [DOI] [PubMed] [Google Scholar]
- 5.Adcock LM, Moore PJ, Schlesinger AE, Armstrong DL. Correlation of ultrasound with postmortem neuropathologic studies in neonates. Pediatr Neurol 1998;19:263–271 [DOI] [PubMed] [Google Scholar]
- 6.Maalouf EF, Duggan PJ, Counsell S, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics 2001;107:719–727 [DOI] [PubMed] [Google Scholar]
- 7.Roelants-Van Rijn AM, Groenendaal F, Beek FJA, Eken P, van Haastert IC, de Vries LS. Parenchymal brain injury in the preterm infant: comparison of cranial ultrasound, MRI and neurodevelopmental outcome. Neuropediatrics 2001;32:80–89 [DOI] [PubMed] [Google Scholar]
- 8.van Wezel-Meijler G, van der Knaap MS, Oosting J, et al. Predictive value of neonatal MRI as compared to ultrasound in premature infants with mild periventricular white matter changes. Neuropediatrics 1999;30:231–238 [DOI] [PubMed] [Google Scholar]
- 9.Childs AM, Cornette L, Ramenghi LA, et al. Magnetic resonance and cranial ultrasound characteristics of periventricular white matter abnormalities in newborn infants. Clin Radiol 2001;56:647–655 [DOI] [PubMed] [Google Scholar]
- 10.Goveart P, De Vries LS. Section V, An Atlas of Neonatal Brain. London: MacKeith Press,1997
- 11.Holling EE, Leviton A. Characteristics of cranial ultrasound white matter echolucencies that predict disability: a review. Dev Med Child Neurol 1998;41:136–139 [DOI] [PubMed] [Google Scholar]
- 12.Schouman-Claeys E, Henry-Feugeas MC, Roset F, et al. Periventricular leukomalacia: correlation between MR imaging and autopsy findings during the 1st 2 months of life. Radiology 1993;189:59–64 [DOI] [PubMed] [Google Scholar]
- 13.Di Pietro MA. The periventricular echogenic “blush” on cranial ultrasonography. AJR Am J Roentgenol 1983;141:851 [Google Scholar]


