Abstract
BACKGROUND AND PURPOSE: Although digital subtraction angiography (DSA) is the reference standard for assessing carotid arteries, it is uncomfortable for patients and has a small risk of disabling stroke and death. These problems have fueled the use of spiral CT angiography and MR angiography. We prospectively compared elliptic centric contrast-enhanced MR angiography and spiral CT angiography with conventional DSA for detecting carotid artery stenosis.
METHODS: Eighty carotid arteries (in 40 symptomatic patients) were assessed. Elliptic centric MR and spiral CT angiographic data were reconstructed with maximum intensity projection and multiplanar reconstruction techniques. All patients had been referred for DSA evaluation on the basis of findings at Doppler sonography, which served as a screening method (degree of stenosis ≥ 70% or inconclusive results). Degree of carotid stenosis estimated by using the three modalities was compared.
RESULTS: Significant correlation with DSA was found for stenosis degree for both elliptic centric MR and spiral CT angiography; however, the correlation coefficient was higher for MR than for CT angiography (r = 0.98 vs r = 0.86). Underestimation of stenoses of 70–99% occurred in one case with elliptic centric MR angiography (a 70% stenosis was underestimated as 65%) and in nine cases with spiral CT angiography, in comparison to DSA findings. Overestimation occurred in two cases with MR angiography (stenoses of 65–67% were overestimated as 70–75%). With CT, overestimation occurred in seven cases; a stenosis of 60% in one case was overestimated as 70%. Both techniques confirmed the three cases of carotid occlusion. With elliptic centric MR angiography, carotid stenoses of 70% or greater were detected with high sensitivity, 97.1%; specificity, 95.2%; likelihood ratio (LR) for a positive test result, 20.4; and ratio of LR+ to LR−, −0.3. With spiral CT angiography, sensitivity, specificity, LR+, and LR+:LR− were 74.3%, 97.6%, 31.2, and 0.3, respectively.
CONCLUSION: Elliptic centric contrast-enhanced MR angiography is more accurate than spiral CT angiography to adequately evaluate carotid stenosis. Furthermore, elliptic centric contrast-enhanced MR angiography appears to be adequate to replace conventional DSA in most patients examined.
Atherosclerosis of the carotid arteries is a major cause of stroke and transient ischemic attack (1). Carotid endarterectomy has been proved to be beneficial in symptomatic patients with a stenosis greater than 70% (2, 3), and even with stenoses of 50–69% (4). Moreover, researchers in the Asymptomatic Carotid Atherosclerosis Study suggested that asymptomatic patients with a stenosis of 60% could benefit from endarterectomy (5). This has prompted a renewed interest in the best method for assessing the degree and length of a carotid stenosis.
Currently, selective digital subtraction angiography (DSA) is widely accepted as the reference standard for assessing the carotid arteries. However, for the patient, conventional DSA is uncomfortable, and there is a small risk of disabling stroke and death (6, 7). The problems associated with selective DSA have fueled the use of alternative and noninvasive techniques, including spiral CT angiography and MR angiography.
Recent study findings have demonstrated the clinical effectiveness of spiral CT angiography in the assessment of narrowing of the lumen of the extracranial carotid artery bifurcation, and CT angiography has already been shown to be sensitive and specific in the evaluation of atherosclerotic stenosis of the carotid artery bifurcation (8–14). Furthermore, 2D and 3D time-of-flight (TOF) MR angiographic images have been reported as useful for accurate evaluation of carotid stenosis (15–18). However, the TOF MR images are flow dependent and therefore analogous to sonographic images. Signal intensity loss occurs with these techniques in the presence of slow flow such as in ulcers or in the carotid bulb. Also, loss of signal intensity can occur in complex or high flow states, such as those that occur with intravoxel dephasing through a high-grade stenosis. The result is that TOF MR angiography has the tendency to overestimate the lesions (19).
More recently, improvements in gradients and sequences have made possible the use of 3D contrast-enhanced MR angiography. The introduction of this technique has increased the confidence in MR angiographic imaging because of better depiction of arterial detail and elimination of many of the TOF MR angiographic artifacts. Another advantage of contrast-enhanced MR angiography is the ability to image from the aortic arch through the intracranial circulation (20–54).
The aim of this study was to prospectively compare spiral CT angiography with elliptic centric contrast-enhanced MR angiography of the carotid arteries by using conventional DSA as the reference standard.
Methods
Patients
We studied 40 consecutive patients (34 men and six women; age range, 42–80 years; mean age, 61.5 years). All patients were referred for conventional DSA evaluation on the basis of Doppler sonography, which served as a screening method (degree of stenosis clearly ≥ 70% or when the results were nonconclusive). Patients were excluded if there was a history of renal disease or allergic reaction to contrast material. We did not use Doppler data because operators were different for each examination. Spiral CT angiography and contrast-enhanced MR angiography were performed the same day; conventional DSA was carried out 2 days later. The study was approved by institutional review board review. Informed consent for spiral CT angiography, elliptic centric contrast-enhanced MR angiography, and selective DSA was obtained from all patients. A total of 80 carotid arteries were assessed in this study.
DSA Technique
All examinations were performed with a Politron 1000 VR unit (Siemens, Nürnberg, Germany). After selective common carotid artery catheterization, obtained by means of a right transfemoral artery puncture, iohexol (Omnigraf; Juste, Madrid, Spain) was injected at 10-mL/s flow rate and included three different projections (posteroanterior, lateral, and 45° oblique views).
MR Angiographic Technique
Elliptic centric contrast-enhanced MR angiography was performed by using a 1.5-T unit (Signa; GE Medical Systems, Milwaukee, WI) with a neurovascular phased-array coil. To calculate the delay time from contrast material injection to the beginning of the sequence, it was necessary to perform a pretest sequence after the injection of a small amount (2 mL) of the Gd-DTPA (Magnograf; Juste, Madrid, Spain). For this, a single-section 2D fast spoiled gradient-recalled acquisition in the steady state (SPGR) sequence, acquired in 1 second, was repeated until an optimal contrast-enhanced image is reached. In our experience, we found a mean delay time of 15 seconds. Then, a 3D fast SPGR sequence with elliptical k-space encoding was acquired in the coronal plane by using the following parameters: TR of 6 msec, minimum TE (the shortest possible TE for the given prescription), 45° flip angle, matrix 320 × 320, section thickness of 1.8 mm interpolated at 0.9 mm (ZIP 2), field of view of 24 cm, acquisition time of 50 seconds. A double dose (0.2 mmol/kg) of the Gd-DTPA was injected into the antecubital vein with a flow rate of 2 mL/s, by using an automatic injector (Spectris; Medrad, Indianola, PA). Postprocessing was performed by using maximum intensity projection for visualization of the entire course of the carotid arteries and the multiplanar reconstruction technique to visualize only a user-specified subvolume (carotid bifurcation) of the volume image.
Spiral CT Angiographic Technique
These images were obtained with a HiSpeed Advantage CT scanner (GE Medical Systems) with a detector configuration of 816 channels and 972 views. Patients were instructed to breathe quietly without swallowing during the scanning period. The imaging volume was determined on a lateral topogram with coverage of approximately 10 cm from the inferior margin of the C6 vertebral body through the C3 vertebral body. Spiral data were acquired in 32 seconds with a section thickness of 2 mm and a table speed of 2 mm/s (210 mA, 120 kV). With a power injection, 150 mL of iopromide (Clarograf; Juste, Madrid, Spain) was injected at a rate of 3 mL/s into an antecubital vein. Administration of each bolus was followed immediately by a 20-mL saline flush. The helical acquisition was begun after the initiation of the bolus administration of contrast material, which was determined by a test of circulation time.
Each carotid artery was segmented by thresholding and visualized with a shaded surface display technique by using a mathematical model of the surface, connecting all pixels with Hounsfield units greater than the predetermined lower threshold. To display only the enhanced vasculature and the calcified structures in the model, the lower threshold of attenuation value was set between 90 and 120 HU. The subsequent removal of vertebral and venous structures was obtained by using a semiautomatic processing technique. The 3D segmented carotid artery was finally visualized by means of maximum intensity projection and shaded surface display techniques, depicting intravascular enhanced voxels and calcifications. The carotid arteries were evaluated in multiple projections by rotating the 3D model to determine the site of maximal stenosis. When calcified plaques or excessively enhanced jugular veins were present, additional information could be obtained from multiplanar volume rendering reconstructions and from the axial images.
Image Analysis
Each imaging technique was performed by one neuroradiologist who was blinded to the results of the other studies. The images were not read at the moment of performing the technique. The measurement of the exact degree of stenosis for each examination was made at the level of maximum stenosis by six neuroradiologists in a randomized order. Distance measurements to quantify stenosis were made with electronic calipers. The six researchers graded the severity of stenosis according to the North American Symptomatic Carotid Endarterectomy Trial, or NASCET, criteria: grade 1, 0–49%; grade 2, 50–69%; grade 3, 70–99%; and grade 4, occlusion (100%). The interobserver concordance was 95% for DSA, 95% for elliptic centric contrast-enhanced MR angiography, and 86% for spiral CT angiography. Disagreements were resolved by discussion. When an internal carotid artery demonstrated a near-complete obstruction with collapse of the lumen (string sign) on the spiral CT angiogram, MR angiogram, or conventional DSA image, the degree of stenosis was defined as 99%. In the other cases, the diameter of the most severe stenosis was divided by the diameter of the distal cervical internal carotid artery beyond the stenosis. Carotid stenoses were measured at the same level on the DSA images, spiral CT angiograms, and elliptic centric MR angiograms. The value was subtracted from 1 and then multiplied by 100 to yield the percentage diameter stenosis.
Images from the three techniques were evaluated for overall quality, including vascular signal intensity, venous suppression, and presence of artifacts. Evaluation criteria for overall quality were 1, excellent; 2, good; 3, moderate; and 4, poor. Furthermore, the amount of calcium on transverse spiral CT angiograms was classified into four categories, depending on the lumen stenosis: type 1, none; type 2, < 50%; type 3, 50–75%; and type 4, 75–100%.
Images from the three techniques were also evaluated for the presence of ulceration. A plaque was classified as ulcerated if it fulfilled the radiographic criteria of an ulcer niche, seen in profile as a crater penetrating into a stenotic plaque, and double opacity on an en face view (the latter criterion applicable for conventional DSA only).
Statistical Analysis
A Pearson rank test was used to find any correlation between findings at DSA and those at spiral CT angiography, as well as those at MR angiography. The values of sensitivity and specificity were established for the presence or absence of stenosis of 70% or greater. The likelihood ratio (LR) for a positive test result (LR+) was calculated by dividing the sensitivity by the false-positive error rate. The LR for a negative test result (LR−) was calculated by dividing the false-negative error rate by the specificity. The ratio of LR+ to LR− (LR+:LR−) was also calculated. The κ statistics were used to test the strength of agreement of each assessment method with DSA. Agreement was classified as mild, κ > 0.40–0.69; good, κ > 0.70–0.89; or excellent, κ > 0.90–1.00.
Results
In all 80 carotid arteries, the quality of both the MR angiograms and DSA images was graded as good or excellent. No relevant motion artifacts diminished the quality of the maximum intensity projection images. In contrast, in 11 of the 80 carotid arteries, the quality of the spiral CT angiograms was graded as moderate because of swallowing artifacts (four cases), calcium excess (five cases), or both (two cases); in the remaining 71 cases, the CT angiograms were graded as good or excellent. Figures 1–4 show various representative examples of this study.
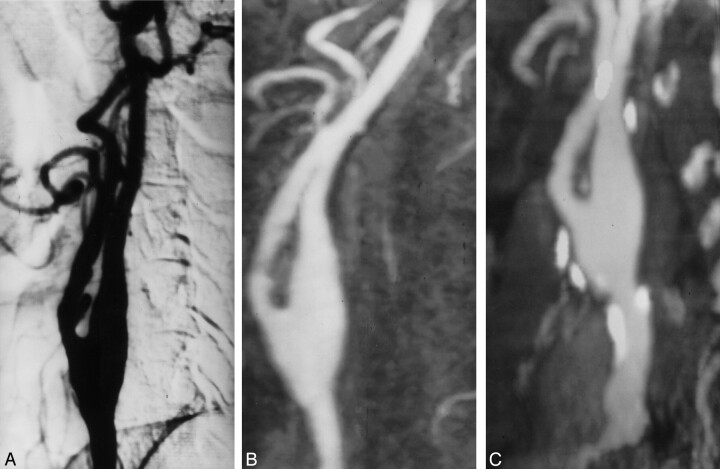
Fig 1.
Grade 1 stenosis of the left internal carotid artery.
A, Oblique DSA image shows minimal stenosis of the left internal carotid artery.
B, Elliptic centric contrast-enhanced MR angiogram, in the same projection as that of the DSA image, depicts a good correlation with DSA.
C, Spiral CT angiogram shows that the calcium distribution (type 1) does not preclude a good evaluation of the vessel.
Fig 4.
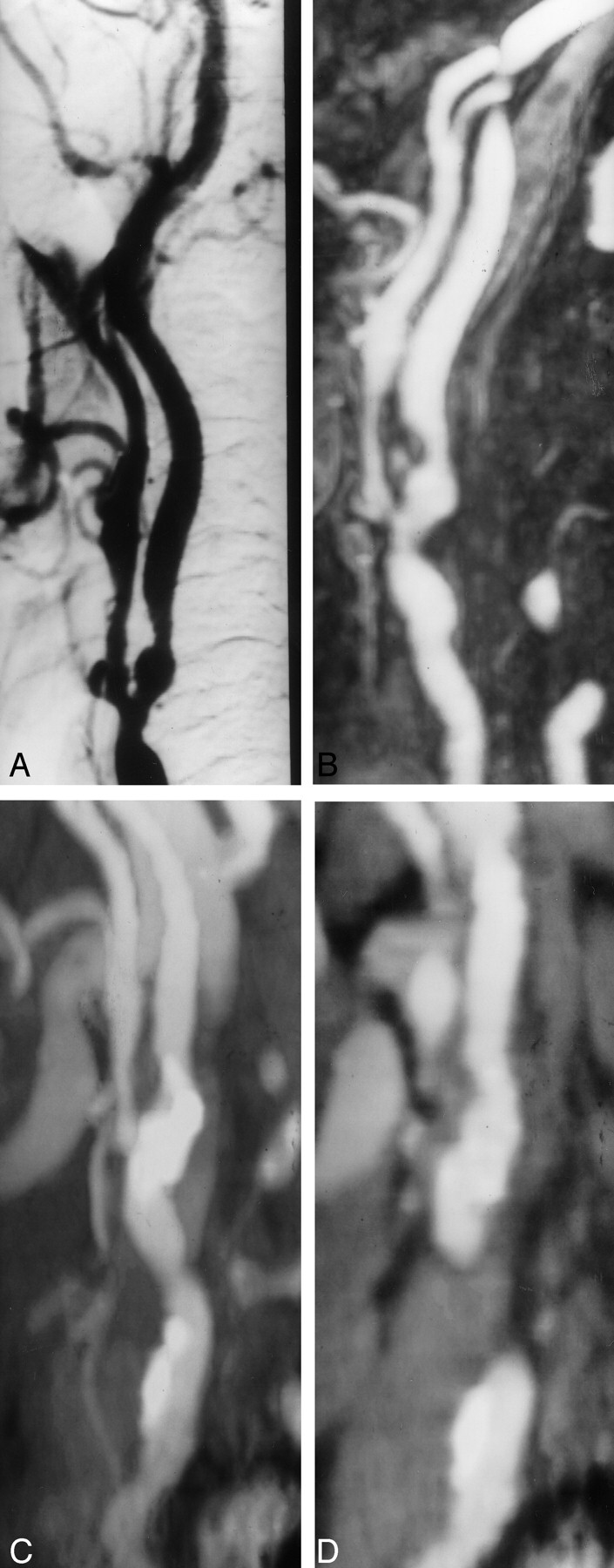
Grade 2 stenosis overestimated with spiral CT angiography.
A, Oblique DSA image shows grade 2 stenosis in the left internal carotid artery.
B, Elliptic centric contrast-enhanced MR angiogram shows the same findings.
C and D, Spiral CT angiograms show type 4 calcification. In this case, the stenosis was overestimated (grade 3) because of the difficulty in separating calcium from the contrast material.
Underestimation of severe stenoses (70–99%) with elliptic centric MR angiography in comparison to DSA findings occurred in one case: a stenosis of 70% was underestimated as 65%. With spiral CT angiography, underestimation of severe stenoses occurred in nine cases. In other words, only one patient had been wrongfully denied surgery with elliptic centric MR angiography in comparison to nine patients with spiral CT angiography. Overestimation with elliptic centric MR angiography occurred in two cases; in both cases, stenoses of 65–67% were overestimated as 70–75% (Fig 2). With spiral CT angiography, overestimation occurred in seven cases; of these, stenosis of 60% in one case was overestimated as 70% (Fig 4) (Table).
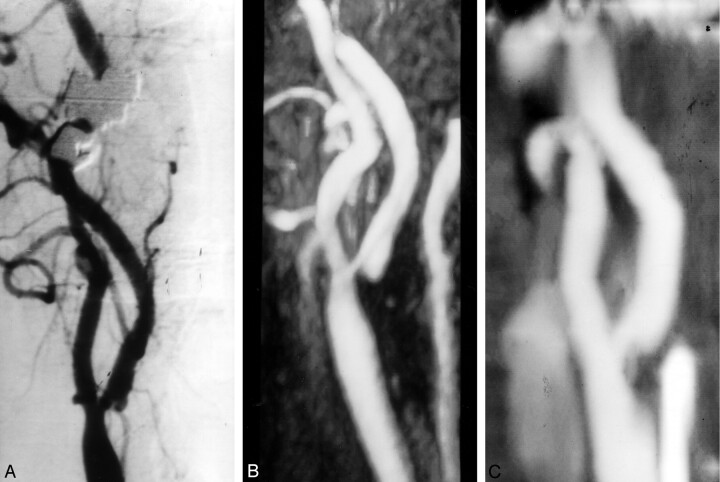
Fig 2.
Grade 2 stenosis overestimated with elliptic centric contrast-enhanced MR angiography.
A, Oblique DSA image demonstrates a grade 2 stenosis in the left internal carotid artery.
B, Elliptic centric contrast-enhanced MR angiogram shows mild overestimation of the stenosis (grade 3).
C, Spiral CT angiogram shows good correlation with DSA.
Comparison of the degree of carotid stenosis with elliptic centric contrast-enhanced MR angiography, spiral CT angiography, and DSA
| Degree of Stenosis | Degree of Stenosis at DSA |
|||
|---|---|---|---|---|
| 0–49% | 50–69% | 70–99% | Occlusion | |
| MR angiography | ||||
| 0–49% | 32 | 0 | 0 | 0 |
| 50–69% | 0 | 8 | 1 | 0 |
| 70–99% | 0 | 2 | 34 | 0 |
| Occlusion | 0 | 0 | 0 | 3 |
| CT angiography | ||||
| 0–49% | 26 | 2 | 1 | 0 |
| 50–69% | 6 | 7 | 8 | 0 |
| 70–99% | 0 | 1 | 26 | 0 |
| Occlusion | 0 | 0 | 0 | 3 |
Note.—Data are number of arteries (n = 80).
The sensitivity of elliptic centric MR angiography in depicting carotid stenoses of 70% or greater was 97.1%; specificity, 95.2%; LR+, 20.4; LR−, 0.03; and LR+:LR−, 680. Moreover, the sensitivity of spiral CT angiography in depicting carotid stenoses of 70% or greater was 74.3%; specificity, 97.6%; LR+, 31.2; LR−, 0.3; and LR+:LR−, 104.
Agreement for classification of degree of stenosis was judged as excellent for elliptic centric MR angiography (κ = 0.95) and good for spiral CT angiography (κ = 0.72). When we divided groups depending on the amount of calcium, the agreements for classification of degree of stenosis were excellent (κ = 0.95) for type 1 (none), good (κ = 0.80) for type 2 (< 50%) and mild (κ = 0.44 and 0.64) for type 3 (50–75%) and 4 (75–100%), respectively.
By using the Pearson rank test, comparison of the percentage carotid artery stenosis determined at DSA with that at elliptic centric MR angiography showed a significant correlation (r = 0.98, P < .001). Likewise, comparison of the percentage carotid artery stenosis determined at DSA with that at spiral CT angiography showed significant correlation (r = 0.86, P < .001).
In the 80 carotid arteries studied with DSA, three carotid occlusions were diagnosed. Elliptic centric MR and spiral CT angiography each confirmed three. Furthermore, among these 80 carotid arteries, conventional DSA depicted five ulcers. All were diagnosed with elliptic centric MR angiography, but only three with spiral CT angiography.
Discussion
Image Quality of MR and CT Angiograms
Elliptic centric contrast-enhanced MR angiograms yielded good to excellent image quality in all examinations. We chose greater spatial resolution at the expense of temporal resolution. In other investigations, a 3D time-resolved imaging of contrast kinetics, or TRICKS, technique was used because of higher temporal resolution (55, 56). This technique has better resolution, has more coverage, and is less sensitive to motion than TOF MR techniques (57). Moreover, this has allowed us to decrease the overall imaging time for carotid studies.
Otherwise, we found that spiral CT angiography had limitations in delineating the lumen of the artery with circumferential wall calcification. Calcifications are the limiting factor on maximum intensity projection images because of the inability to differentiate mural calcifications and intramural contrast material (Fig 4). To minimize this limitation, analysis in conjunction with the transverse source images may be useful (58). Although transverse source images, including multiplanar volume rendering images, were also analyzed in this study, dense circumferential calcification of the arterial wall caused artifacts that interfered with the evaluation of the degree of stenosis. Indeed, we tried unsuccessfully to remove calcifications by using sophisticated software (59). In these cases, circumferential calcified plaque surrounded by a high-attenuation rim owing to partial volume effects could not permit the visualization of the contrast material-filled lumen in transparency through the calcification. The brightness of this rim was similar to that observed for contrast material within the vessel lumen. Therefore, measuring the apparent vessel lumen resulted in underestimation of the carotid stenosis. In this sense, when the amount of calcium was less than 50% or was absent, the agreements for classification of degree of stenosis were judged as good and excellent, respectively. Because elliptic centric MR angiography more correctly revealed the calcified stenotic lumens than did spiral CT angiography, we believe that it will be more useful for the evaluation of the lumina of vessels with circumferential calcification.
Efficacy of MR versus CT Angiography
Good correlation of elliptic centric contrast-enhanced MR angiography with conventional DSA in depicting carotid stenosis has been reported (33, 51–54). According to those studies, the sensitivity of elliptic centric MR angiography for the identification of surgical lesions (ie, carotid stenosis of 70% or greater) has been found to be consistently high at 93–100%, with a specificity of 85–100%. In our study, the diagnostic accuracy of elliptic centric MR angiography for depicting diseased vessels was similar to these previous results.
The diagnostic accuracy of spiral CT angiography has been validated in a number of studies comparing this technique with DSA. The sensitivity and specificity values of spiral CT angiography ranged from 88% to 100% and 83% to 100%, respectively (10, 53, 58–61). However, our results were poorer, being sensitivity 74.3% and specificity 97.6%. As mentioned, this is likely in relation to the poor quality of several images owing to swallowing artifacts and calcifications.
Spiral CT angiography has been compared with contrast-enhanced MR angiography for the detection and quantification of carotid stenosis in only one study (53). In that study of 44 arteries, spiral CT angiography, contrast-enhanced MR angiography, and DSA closely correlated in the evaluation of the degree of stenosis. In our study, correlation between spiral CT angiography and DSA was poorer than that of elliptic centric contrast-enhanced MR angiography with DSA (0.86 vs 0.98). Moreover, the sensitivity of spiral CT angiography in depicting carotid stenoses of 70% or greater was lower than that of elliptic centric contrast-enhanced MR angiography. Taken together, the results of our study suggest that elliptic centric MR angiography has more advantages than does spiral CT angiography. Furthermore, one should bear in mind the intrinsic disadvantages of spiral CT angiography, including the need for ionizing radiation, iodinated contrast material, optimization of imaging delay time, and careful evaluation of the superimposition of bone and venous structures.
Depicting Occlusions and Ulcers
For carotid occlusions, the small number of cases (n=3) we had did not enable us to reach a conclusion. However, the cases we did evaluate suggest a high accuracy for both elliptic centric contrast-enhanced MR angiography and spiral CT angiography for diagnosis of carotid occlusion. The ability to depict occlusions accurately is important because a patient with a nearly completely obstructed artery would be suitable for carotid endarterectomy, whereas a patient with a carotid occlusion would not.
Plaque morphology is being increasingly recognized as an independent risk factor for developing stroke. Specifically, necrotic and ulcerated plaques appear to be associated with increased risk of embolic event (62). With elliptic centric MR angiography, all the ulcerated plaques were detected in this study. In contrast, spiral CT angiography enabled detection of only three of the five plaques; this finding has been observed by other authors (63, 64).
Limitations of This Study
This study had some limitations. First, we had some selection bias; therefore, the final sensitivity and specificity may not reflect the true values for an unselected population. We chose to examine only participants with stenoses of 70% or greater, as revealed at Doppler sonography. This could explain the fact that a great number of cases had important calcifications.
Second, the choice of DSA as the reference standard may be considered as a limitation of our study. We chose conventional DSA because international randomized trials have used and still use this technique as the reference standard. However, one should keep in mind that conventional DSA also has limitations. In this sense, rotational angiography frequently depicts more severe internal carotid artery stenosis than does conventional DSA (65). Moreover, the images generally provided by conventional DSA are not suitable enough to accurately quantify the degree of stenosis, since eccentric plaques cause an oval appearance of the lumen, which, in the case of radiographs not perpendicular to the borders, underestimates the stenosis (66, 67). Therefore, it is not surprising to find, in our study, overestimation with elliptic centric MR angiography, which always quantifies stenosis through the ideal angle. This bias could have been avoided if we had considered endarterectomy plaques as the reference standard, as Pan et al (68) did. However, as these authors stated, this method cannot be retained as a reference because the lack of arterial pressure likely modifies the measures, and the internal carotid artery is not measurable 1 cm past the end of the bulb.
Third, our CT scanner was a single-detector row unit. Multi-detector row helical CT offers a two- to threefold improvement in volume coverage speed by reducing scanning time by one-half to one-third while preserving the image quality provided by single-detector row helical CT. These advantages could translate into a substantial increase in the region scanned without additional injection of contrast material, better separation of the arterial and venous phases in multiphase data acquisitions, and substantially higher quality of reconstructed 3D data because of improvements in z-axis resolution (69).
Conclusions
Our results yield two conclusions. First, elliptic centric contrast-enhanced MR angiography is more accurate than spiral CT angiography to adequately evaluate carotid stenosis, especially when there is an excess of calcium. Second, we found an excellent statistical correlation between elliptic centric MR angiography and DSA. Therefore, elliptic centric MR angiography appears to be adequate to replace conventional DSA in most patients examined. We believe that DSA needs be considered only in claustrophobic patients or those with contraindications to MR (ie, pacemakers or metal devices) and in those studies that disclose stenosis around 70%.
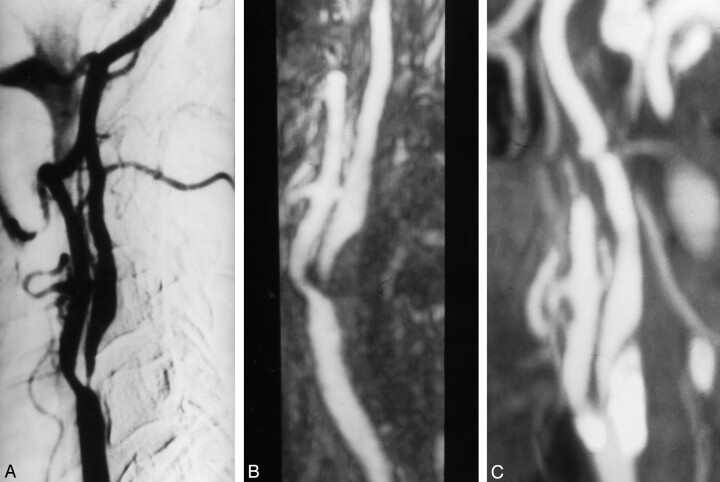
Fig 3.
Grade 4 stenosis with good correlation in all techniques.
A, Oblique DSA image shows critical stenosis in the right internal carotid artery.
B, Elliptic centric contrast-enhanced MR angiogram depicts the same information.
C, Spiral CT angiogram shows a good correlation with the other imaging techniques. Notice a type 3 calcification in the wall of artery; however, it is sufficiently separated in the reconstruction image.
Acknowledgments
The authors gratefully acknowledge the vital help of Drs. A. Ramos, J. Porto, A. Gómez de la Cámara, F. Herráiz, R. Wert, and A. Vega.
Footnotes
Supported in part by grants from Sanofi, Barcelona, Spain, and Juste, Madrid, Spain.
Presented at the 39th annual meeting of the American Society of Neuroradiology, Boston, April 2001.
References
- 1.Seeger MD, Barratt BS, Lawson GA, Klingman N. The relationship between carotid plaque composition, morphology, and neurological symptoms. J Surg Res 1995;58:330–336 [DOI] [PubMed] [Google Scholar]
- 2.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–453 [DOI] [PubMed] [Google Scholar]
- 3.European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis.Lancet 1991;337:1235–1243 [PubMed] [Google Scholar]
- 4.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis: North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415–1425 [DOI] [PubMed] [Google Scholar]
- 5.Executive Committee for the Asymptomatic Carotid Atherosclerotic Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273:1421–1428 [PubMed] [Google Scholar]
- 6.Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke 1990;21:209–222 [DOI] [PubMed] [Google Scholar]
- 7.Waugh JR, Sacharias N. Arteriographic complications in the DSA era. Radiology 1992;182:243–246 [DOI] [PubMed] [Google Scholar]
- 8.Schwartz RB, Jones KM, Chernoff DM, et al. Common carotid artery bifurcation: evaluation with spiral CT. Radiology 1992;185:513–519 [DOI] [PubMed] [Google Scholar]
- 9.Marks MP, Napel S, Jordan JE, Enzmann DR. Diagnosis of carotid artery disease: preliminary experience with maximum-intensity-projection spiral CT angiography. AJR Am J Roentgenol 1993;160:1267–1271 [DOI] [PubMed] [Google Scholar]
- 10.Cumming MJ, Morrow IM. Carotid artery stenosis: a prospective comparison of CT angiography and conventional angiography. AJR Am J Roentgenol 1994;163:517–523 [DOI] [PubMed] [Google Scholar]
- 11.Link J, Brossmann J, Grabener M, et al. Spiral CT angiography and selective digital subtraction angiography of internal carotid artery stenosis. AJNR Am J Neuroradiol 1996;17:89–94 [PMC free article] [PubMed] [Google Scholar]
- 12.Cinat M, Lane CT, Pham H, Lee A, Wilson SE, Gordon I. Helical CT angiography in the preoperative evaluation of carotid artery stenosis. J Vasc Surg 1998;28:290–230 [DOI] [PubMed] [Google Scholar]
- 13.Goddard AJ, Mendelow AD, Birchall D. Computed tomography angiography in the investigation of carotid stenosis. Clin Radiol 2001;56:523–534 [DOI] [PubMed] [Google Scholar]
- 14.Marcus CD, Ladam-Marcus VJ, Bigot JL, Clement C, Baehrel B, Menanteau BP. Carotid arterial stenosis: evaluation at CT angiography with the volume-rendering technique. Radiology 1999;211:775–780 [DOI] [PubMed] [Google Scholar]
- 15.Laster RE, Acker JD, Halford HH, et al. Assessment of MRA vs angiography for evaluation of cervical carotid bifurcation disease. AJNR Am J Neuroradiol 1993;14:681–688 [PMC free article] [PubMed] [Google Scholar]
- 16.Scarabino T, Carriero A, Magarelli N, et al. MR angiography in carotid stenosis: a comparison of three techniques. Eur J Radiol 1998;28:117–125 [DOI] [PubMed] [Google Scholar]
- 17.Magarelli N, Scarabino T, Simeone AL, et al. Carotid stenosis: a comparison between MR and spiral CT angiography. Neuroradiology 1998;40:367–373 [DOI] [PubMed] [Google Scholar]
- 18.Westwood ME, Kelly S, Berry E, et al. Use of magnetic resonance angiography to select candidates with recently symptomatic carotid stenosis for surgery: systematic review. BMJ 2002;324:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoogeveen RM, Bakker CJ, Viergever MA. Limits to the accuracy of vessel diameter measurement in MR angiography. J Magn Reson Imaging 1998;8:1228–1235 [DOI] [PubMed] [Google Scholar]
- 20.Levy RA, Prince MR. Arterial-phase three-dimensional contrast-enhanced MR angiography of the carotid artery. AJR Am J Roentgenol 1996;167:211–215 [DOI] [PubMed] [Google Scholar]
- 21.Cloft HJ, Murphy KJ, Prince MR, Brunberg JA. 3D gadolinium-enhanced MR angiography of the carotid arteries. Magn Reson Imaging 1996;14:593–600 [DOI] [PubMed] [Google Scholar]
- 22.Krinsky G, Rofsky N, Flyer M, et al. Gadolinium-enhanced three-dimensional MR angiography of acquired arch vessel disease. AJR Am J Roentgenol 1996;167:981–987 [DOI] [PubMed] [Google Scholar]
- 23.Remonda L, Heid O, Schroth G. Carotid artery stenosis, occlusion, and pseudo-occlusion: first-pass, gadolinium-enhanced, three-dimensional MR angiography—preliminary study. Radiology 1998;209:95–102 [DOI] [PubMed] [Google Scholar]
- 24.Slosman F, Stolpen AH, Lexa FJ, et al. Extracranial atherosclerotic carotid artery disease: evaluation of non-breath-hold three-dimensional gadolinium-enhanced MR angiography. AJR Am J Roentgenol 1998;170:489–495 [DOI] [PubMed] [Google Scholar]
- 25.Willig DS, Turski PA, Frayne R, et al. Contrast-enhanced 3D MR DSA of the carotid artery bifurcation: preliminary study of comparison with unenhanced 2D and 3D time-of-flight MR angiography. Radiology 1998;208:447–451 [DOI] [PubMed] [Google Scholar]
- 26.Leclerc X, Martinat P, Godefroy O, et al. Contrast-enhanced three-dimensional fast imaging with steady-state precession (FISP) MR angiography of supraaortic vessels: preliminary results. AJNR Am J Neuroradiol 1998;19:1405–1413 [PMC free article] [PubMed] [Google Scholar]
- 27.Isoda H, Takehara Y, Isogai S, et al. Technique for arterial-phase contrast-enhanced three-dimensional MR angiography of the carotid and vertebral arteries. AJNR Am J Neuroradiol 1998;19:1241–1244 [PMC free article] [PubMed] [Google Scholar]
- 28.Steffens JC, Link J, Heller M. Contrast-enhanced magnetic resonance angiography of the cervical arteries. Invest Radiol 1998;33:573–577 [DOI] [PubMed] [Google Scholar]
- 29.Krinsky G, Maya M, Rofsky N, et al. 3D gadolinium-enhanced MRA of the aortic arch vessels in the detection of atherosclerotic cerebrovascular occlusive disease. J Comput Assist Tomogr 1998;22:167–178 [DOI] [PubMed] [Google Scholar]
- 30.Kim JK, Farb RI, Wright GA. Test bolus examination in the carotid artery at dynamic gadolinium-enhanced MR angiography. Radiology 1998;206:283–289 [DOI] [PubMed] [Google Scholar]
- 31.Martinat P, Leclerc X, Gauvrit JY, Giboreau F, Pruvo JP. Contribution of fast-sequence three-dimensional MRI angiography with gadolinium injection in the evaluation of supra-aortic vessels. J Radiol 1998;79:673–680 [PubMed] [Google Scholar]
- 32.Cloft HJ, Kallmes DF. Gadolinium-enhanced MR angiography for carotid artery disease. AJR Am J Roentgenol 1999;172:833–834 [DOI] [PubMed] [Google Scholar]
- 33.Scarabino T, Carriero A, Giannatempo GM, et al. Contrast-enhanced MR angiography (CE MRA) in the study of the carotid stenosis: comparison with digital subtraction angiography (DSA). Neuroradiology 1999;26:87–91 [PubMed] [Google Scholar]
- 34.Leclerc X, Gauvrit JY, Nicol L, Pruvo JP. Contrast-enhanced MR angiography of the craniocervical vessels: a review. Neuroradiology 1999;41:867–874 [DOI] [PubMed] [Google Scholar]
- 35.Sardanelli F, Zandrino F, Parodi RC, De Caro G. MR angiography of internal carotid arteries: breath-hold Gd-enhanced 3D fast imaging with steady-state precession versus unenhanced 2D and 3D time-of-flight techniques. J Comput Assist Tomogr 1999;23:208–215 [DOI] [PubMed] [Google Scholar]
- 36.Sardanelli F, Zandrino F, Parodi RC, De Caro G. MR angiography of internal carotid arteries: breath-hold Gd-enhanced 3D fast imaging with steady-state precession versus unenhanced 2D and 3D time-of-flight techniques. J Comput Assist Tomogr 1999;23:208–215 [DOI] [PubMed] [Google Scholar]
- 37.Leclerc X, Gauvrit JY, Nicol L, Martinat P, Pruvo JP. Gadolinium-enhanced fast three-dimensional angiography of the neck: technical aspect. Invest Radiol 1999;34:204–210 [DOI] [PubMed] [Google Scholar]
- 38.Kollias SS, Binkert CA, Ruesch S, Valavanis A. Contrast-enhanced MR angiography of the supra-aortic vessels in 24 seconds: a feasibility study. Neuroradiology 1999;41:391–400 [DOI] [PubMed] [Google Scholar]
- 39.Huston J 3rd, Fain SB, Riederer SJ, Wilman AH, Bernstein MA, Busse RF. Carotid arteries: maximizing arterial to venous contrast in fluoroscopically triggered contrast-enhanced MR angiography with elliptic centric view ordering. Radiology 1999;211:265–273 [DOI] [PubMed] [Google Scholar]
- 40.Korosec FR, Turski PA, Carroll TJ, Mistretta CA, Grist TM. Contrast-enhanced MR angiography of the carotid bifurcation. J Magn Reson Imaging 1999;10:317–325 [DOI] [PubMed] [Google Scholar]
- 41.Melhem ER, Caruthers SD, Faddoul SG, Tello R, Jara H. Use of three-dimensional MR angiography for tracking a contrast bolus in the carotid artery. AJNR Am J Neuroradiol 1999;20:263–266 [PMC free article] [PubMed] [Google Scholar]
- 42.Leclerc X, Lucas C, Godefroy O, et al. Preliminary experience using contrast-enhanced MR angiography to assess vertebral artery structure for the follow-up of suspected dissection. AJNR Am J Neuroradiol 1999;20:1482–1490 [PMC free article] [PubMed] [Google Scholar]
- 43.Kaandorp DW, Kopinga K, Wijn PF. Venous signal suppression in 3D dynamic Gd-enhanced carotid artery imaging using the eigenimage filter. Magn Reson Med 1999;42:307–313 [DOI] [PubMed] [Google Scholar]
- 44.Leclerc X, Nicol L, Gauvrit JY, Le Thuc V, Leys D, Pruvo JP. Contrast-enhanced MR angiography of supraaortic vessels: the effect of voxel size on image quality. AJNR Am J Neuroradiol 2000;21:1021–1027 [PMC free article] [PubMed] [Google Scholar]
- 45.Isoda H, Takehara Y, Isogai S, et al. Software-triggered contrast-enhanced three-dimensional MR angiography of the intracranial arteries. AJR Am J Roentgenol 2000;174:371–375 [DOI] [PubMed] [Google Scholar]
- 46.Aoki S, Nakajima H, Kumagai H, Araki T. Dynamic contrast-enhanced MR angiography and MR imaging of the carotid artery: high-resolution sequences in different acquisition planes. AJNR Am J Neuroradiol 2000;21:381–385 [PMC free article] [PubMed] [Google Scholar]
- 47.Fellner FA, Fellner C, Wutke R, et al. Fluoroscopically triggered contrast-enhanced 3D MR DSA and 3D time-of-flight turbo MRA of the carotid arteries: first clinical experiences in correlation with ultrasound, x-ray angiography, and endarterectomy findings. Magn Reson Imaging 2000;18:575–585 [DOI] [PubMed] [Google Scholar]
- 48.Melhem ER, Serfaty JM, Jones L, et al. Contrast-enhanced MR angiography: the effects of k-space truncation on luminal representation in a carotid artery phantom model. AJNR Am J Neuroradiol 2000;21:1028–1031 [PMC free article] [PubMed] [Google Scholar]
- 49.Serfaty JM, Chirossel P, Chevallier JM, Ecochard R, Froment JC, Douek PC. Accuracy of three-dimensional gadolinium-enhanced MR angiography in the assessment of extracranial carotid artery disease. AJR Am J Roentgenol 2000;175:455–463 [DOI] [PubMed] [Google Scholar]
- 50.Jager HR, Moore EA, Bynevelt M, et al. Contrast-enhanced MR angiography in patients with carotid artery stenosis: comparison of two different techniques with an unenhanced 2D time-of-flight sequence. Neuroradiology 2000;42:240–248 [DOI] [PubMed] [Google Scholar]
- 51.Huston J 3rd, Fain SB, Wald JT, et al. Carotid artery: elliptic centric contrast-enhanced MR angiography compared with conventional angiography. Radiology 2001;218:138–143 [DOI] [PubMed] [Google Scholar]
- 52.Carr JC, Shaibani A, Russell E, Finn JP. Contrast-enhanced magnetic resonance angiography of the carotid circulation. Top Magn Reson Imaging 2001;12:349–357 [DOI] [PubMed] [Google Scholar]
- 53.Randoux B, Marro B, Koskas F, et al. Carotid artery stenosis: prospective comparison of CT, three-dimensional gadolinium-enhanced MR, and conventional angiography. Radiology 2001;220:179–185 [DOI] [PubMed] [Google Scholar]
- 54.Remonda L, Senn P, Barth A, Arnold M, Lövblad KO, Schroth G. Contrast-enhanced 3D MR angiography of the carotid artery: comparison with conventional digital subtraction angiography. AJNR Am J Neuroradiol 2002;23:213–219 [PMC free article] [PubMed] [Google Scholar]
- 55.Korosec FR, Frayne R, Grist TM, Mistretta CA. Time-resolved contrast-enhanced 3D MR angiography. Magn Reson Med 1996;36:345–351 [DOI] [PubMed] [Google Scholar]
- 56.Naganawa S, Koshikawa T, Fukatsu H, et al. Contrast-enhanced MR angiography of the carotid artery using 3D time-resolved imaging of contrast kinetics: comparison with real-time fluoroscopic triggered 3D-elliptical centric view ordering. Radiat Med 2001;19:185–192 [PubMed] [Google Scholar]
- 57.De Marco JK, Schonfeld S, Keller I, Bernstein MA. Contrast-enhanced carotid MR angiography with commercially available triggering mechanisms and elliptic centric phase encoding. AJR Am J Roentgenol 2001;176:221–227 [DOI] [PubMed] [Google Scholar]
- 58.Dillon EM, Van Leeuwen MS, Fernandez MA. CT angiography: application to evaluation of carotid artery stenosis. Radiology 1993;189:211–219 [DOI] [PubMed] [Google Scholar]
- 59.Simeone A, Carriero A, Armillotta M, et al. Spiral CT angiography in the study of the carotid stenoses. J Neuroradiol 1997;24:18–22 [PubMed] [Google Scholar]
- 60.Leclerc X, Godefroy O, Lucas C, et al. Internal carotid arterial stenosis: CT angiography with volume rendering. Radiology 1999;210:673–682 [DOI] [PubMed] [Google Scholar]
- 61.Berg MH, Manninen HI, Rasanen HT, Vanninen RL, Jaakkola PA. CT angiography in the assessment of carotid artery atherosclerosis. Acta Radiol 2002;43:116–124 [DOI] [PubMed] [Google Scholar]
- 62.Hatsukami TS, Ferguson MS, Beach KW, et al. Carotid plaque morphology and clinical events. Stroke 1997;28:95–100 [DOI] [PubMed] [Google Scholar]
- 63.Oliver TB, Lammie GA, Wright AR, et al. Atherosclerotic plaque at the carotid bifurcation: CT angiographic appearance with histopathologic correlation. AJNR Am J Neuroradiol 1999;20:897–901 [PMC free article] [PubMed] [Google Scholar]
- 64.Walker LJ, Ismail A, McMeekin W, Lambert D, Mendelow AD, Birchall D. Computed tomography angiography for the evaluation of carotid atherosclerotic plaque: correlation with histopathology of endarterectomy specimens. Stroke 2002;33:977–981 [DOI] [PubMed] [Google Scholar]
- 65.Elgersma OE, Buijs PC, Wust AF, van der Graaf Y, Eikelboom BC, Mali WP. Maximum internal carotid arterial stenosis: assessment with rotational angiography versus conventional intraarterial digital subtraction angiography. Radiology 1999;213:777–783 [DOI] [PubMed] [Google Scholar]
- 66.Polak JF. Noninvasive carotid evaluation: carpe diem. Radiology 1993;186:329–331 [DOI] [PubMed] [Google Scholar]
- 67.Vanninen R, Manninen H, Koivisto K, Tulla H, Partanen K, Puranen M. Carotid stenosis by digital subtraction angiography: reproducibility of the European Carotid Surgery Trial measurement methods and trials. AJNR Am J Neuroradiol 1994;15:1635–1641 [PMC free article] [PubMed] [Google Scholar]
- 68.Pan XM, Saloner D, Reilly LM, et al. Assessment of carotid artery stenosis by ultrasonography, conventional angiography, and magnetic resonance angiography: correlation with ex vivo measurement of plaque stenosis. J Vasc Surg 1995;21:82–88 [DOI] [PubMed] [Google Scholar]
- 69.Hu H, He HD, Foley WD, Fox SH. Multidetector-row helical CT: image quality and volume coverage speed. Radiology 2000;215:55–62 [DOI] [PubMed] [Google Scholar]