Abstract
Summary: Pituitary duplication is a rare malformation, reported previously in approximately 18 patients. It is usually unsuspected before imaging, although it occurs most commonly in association with complicated midline and skull base anomalies. It is easily shown by MR imaging. Five new cases of pituitary duplication were diagnosed by using MR imaging studies reviewed at the Hospital for Sick Children. Among the many associated midline abnormalities, partial basilar artery duplication is a previously undescribed finding that we observed in all our cases. Cases of basilar artery duplication or fenestration are associated with altered flow dynamics, leading to a higher incidence of aneurysms. Periodic surveillance for this potential complication may be warranted.
We report a rare congenital anomaly, basilar artery segmentation, occurring in five new cases of duplication of the pituitary gland. This feature has not been previously reported in the literature. We discuss the embryology and potential complications of basilar artery and pituitary duplication and review the related literature.
Cases
Five new cases of pituitary duplications were diagnosed based on MR imaging studies reviewed at the Hospital for Sick Children, Toronto, Canada. The patients included a 28-week-premature female, a 2-month-old female, a 2-day-old male, a 3-year-old male, and a neonate male patient. Initial reasons for imaging included facial dysmorphism, including oral mass, developmental delay, and, in one patient, prematurity-related complications. No patient had symptoms referable to the pituitary axis at the time of imaging, although one of the four survivors developed precocious puberty. Routine imaging of the brain led to dedicated sellar imaging. In all patients, the basilar artery was well seen on axial and coronal view spin-echo images. Three patients also underwent 3D time-of-flight MR angiography to evaluate the circle of Willis.
Case 1
A 1-year-old male patient was referred for MR imaging of the head because of macrocephaly (head circumference beyond the 98th percentile), mild facial dysmorphism, and mild development delay. On the localizer sagittal view MR images, thickening of the hypothalamus was observed, which led us to perform dedicated MR imaging of the sella with thin section coronal view images. The MR imaging revealed two pituitary glands with two infundibular stalks (Fig 1). The tuber cinereum and mamillary bodies were fused into one thickened mass (tubomamillary fusion), extending along the floor of the third ventricle. Mild hypertelorism was present. Also noted was a wide pons with a prominent ventral raphe and a sagittal cleft in the odontoid. 3D time-of-flight sequence MR angiography revealed duplication of the basilar artery from the level of the midpons rostrally. The posterior cerebral artery and the superior cerebellar artery on each respective side arose separately from the split basilar artery. The circle of Willis was otherwise normal. The results of metabolic, chromosomal, and hormonal studies were normal. No laboratory evidence of pituitary dysfunction was observed.
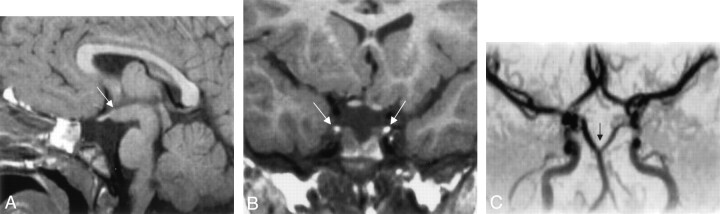
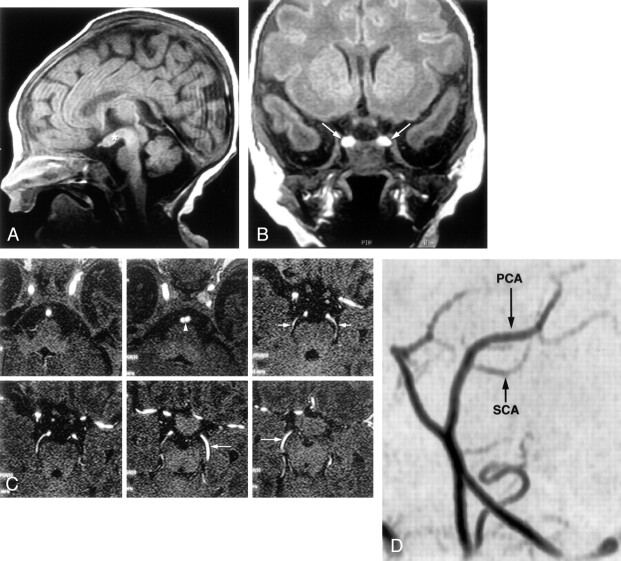
Fig 1.
Images from the case of a 1-year-old male patient with macrocephaly and mild facial dysmorphism (case 1).
A, Sagittal view T1-weighted MR image shows tubomammillary fusion (arrow).
B, Coronal view T1-weighted MR image shows the bright signal intensity in the posterior pituitary of a duplicated gland.
C, Frontal projection time-of-flight MR angiogram shows the distal split of the basilar artery (arrow).
Case 2
A 2-day-old female patient who was born at term was referred for MR imaging to evaluate a protruding whitish mass from the oral cavity. This was thought to be a meningocele or an encephalocele, because the mass increased in size or protruded more when the patient cried. No other abnormal clinical findings were observed. MR imaging showed tubomamillary fusion, two infundibular recesses, two pituitary stalks, and two bright pituitary glands. There is increased signal intensity of the pituitary gland due to hormonal influences, a known feature in the first 6 weeks of life. This finding made the diagnosis of pituitary duplication obvious. Associated corpus callosal agenesis was also noted in this case. The mass in the oral cavity was seen to be a lipomatous mass (bright on T1-weighted images and dark on T2-weighted images) and was attached to the soft palate. MR angiograms were not available for review in this case. Coronal view fast spin-echo T2-weighted images revealed a partial segmentation of the basilar artery (similar to that observed in case 1) from the level of midpons rostrally. CT with 3D reconstructions showed duplication of the bony sella (Fig 2). Hormonal and metabolic studies did not reveal any abnormalities. The oral mass was excised and reported to be a dermoid.
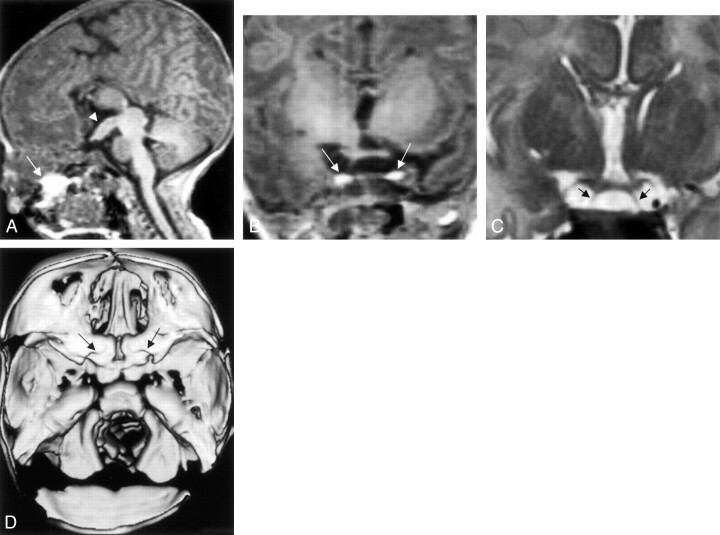
Fig 2.
Images from the case of a 2-day-old female patient who presented with a protruding whitish mass from the oral cavity (case 2).
A, Sagittal view T1-weighted MR image shows tubomammillary fusion (short arrow) and the oral dermoid (long arrow).
B, Coronal view T1-weighted MR image shows duplicated pituitary gland (arrows).
C, Coronal view T2-weighted MR image shows two infundibular stalks (arrows).
D, 3D CT scan shows duplicated sella.
Case 3
This neonate female patient initially underwent CT at birth to assess a craniofacial abnormality, which was subsequently diagnosed as mandibular duplication and teratoma. She underwent bilateral sagittal split advancement to correct spheno-mandibular-maxillary fusion. MR imaging was performed when the patient was 2 months old and showed two pituitary glands and two infundibula. Hypothalamic mass or thickening due to tubomammillary fusion was also evident. In this patient, too, similar segmentation of the basilar artery was noted from the midpons rostrally (Fig 3). The patient, who was 8 years old at the time of this writing, subsequently developed precocious puberty and is being treated with intramuscular injection of DepoLupron (7.5 mg administered once a month).
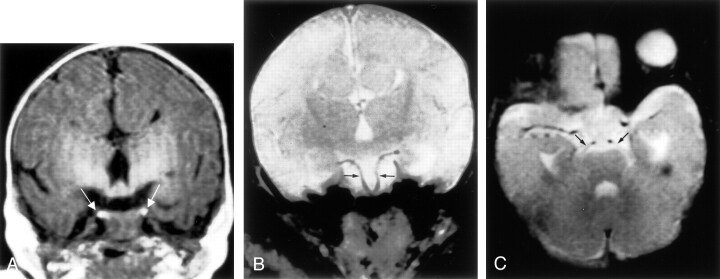
Fig 3.
Images from the case of a 1-day-old female patient with craniofacial deformity (case 3).
A, Coronal view T1-weighted MR image shows duplicated pituitary gland (arrows).
B, Coronal view T2-weighted MR image shows duplicated basilar artery (arrows).
C, Axial view T2-weighted MR image shows each superior cerebellar artery (arrows) arising separately from the duplicated basilar artery.
Case 4
Sonography of the brain of this 28-week-premature female patient showed enlarged ventricles with germinal matrix and intraventricular blood. The patient underwent CT to further evaluate this finding. Anomalies of the skull base suggesting sellar duplication were identified, and MR imaging was subsequently performed. The MR imaging confirmed enlarged ventricles with intraventricular blood. Two pituitary glands were seen and were bright on T1-weighted images. Hypothalamic thickening was also obvious on MR images. The spinal cord in the superior cervical region was tethered anteriorly into the pharynx through split vertebral bodies. MR angiography showed partial duplication of the basilar artery (Fig 4). The patient died shortly thereafter as a result of complications of prematurity. At autopsy, the floor of the third ventricle was thickened with fusion of the tuber cinereum and the mammillary bodies. Basilar artery duplication was confirmed at autopsy. Each pituitary gland was normal in its morphology and histology.
Fig 4.
Images from the case of a premature female patient who was born at 28 weeks (case 4).
A, Coronal view T1-weighted MR image shows duplicated pituitary gland (long arrows). Short arrow indicates germinal matrix bleed on the right side.
B, Time-of-flight MR angiogram shows duplicated distal basilar artery.
C, Autopsy probe points to the origin of the duplicated distal basilar artery. Note the thickened hypothalamus.
Case 5
This neonate male patient initially underwent CT to assess difficulty in passing a nasal feeding tube and suspected choanal atresia. CT showed an oral dermoid and widened pituitary fossa. Identification of a distal split in the basilar artery shown on CT scans led us to suggest pituitary duplication, which was confirmed by MR imaging (Fig 5). The mass in the midline in the palate was excised and was reported to be a “hairy” dermoid. MR angiography confirmed basilar artery duplication, and the lower brain stem in this case, too, was tethered anteriorly.
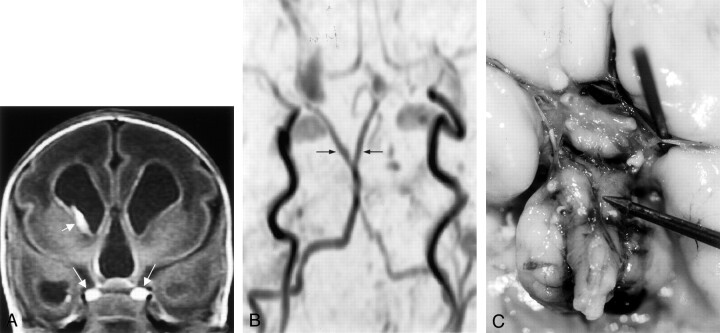
Fig 5.
Images from the case of a 2-day-old female patient with suspected choanal atresia (case 5).
A, Sagittal view T1-weighted image shows characteristic tubomammillary fusion (asterisk).
B, Coronal view T1-weighted image shows the bright duplicated pituitary gland (arrows).
C, Source MR angiograms obtained through the vertebrobasilar system. Arrowhead indicates duplicated basilar artery. Short arrows indicate superior cerebellar arteries arising from duplicated basilar artery. Long arrows indicate posterior cerebral artery.
D, Oblique view MR angiogram shows the superior cerebellar arteries (SCA) originating from the distal duplicated basilar artery. PCA, posterior cerebral artery.
Summary of Imaging Findings
Pituitary Gland, Floor of the Third Ventricle
Fusion of the mammillary bodies and tuber cinereum into a single midline mass was identified in all patients (Table 1). The resultant thickened floor of the third ventricle was easily identified on sagittal view images and let to dedicated sellar imaging. In all patients, duplication of the pituitary stalk and gland was present. Pituitary glands were particularly easy to identify on the MR images of the three neonates because the pituitary gland at that age is homogenously bright on T1-weighted images and because of a lack of fatty marrow in the sphenoid gland.
TABLE 1:
Pituitary duplications
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Sex, age | Male, 1 year | Female, 2 days | Female, 1 day | Female, 28 weeks’ gestation | Female, 2 days |
| Clinical presentation | Macrocephaly, mild facial dysmorphism, developmental delay | Protruding whitish mass from oral cavity | Craniofacial deformity | Prematurity, hydrocephalus with intraventricular hemorrhage on ultrasonogram | Suspected choanal atresia |
| Pituitary gland, duplicated pituitary and stalk | Yes | Yes | Yes | Yes | Yes |
| Hypothalamic thickening and tubomammillary fusion | Yes | Yes | Yes | Yes | Yes |
| Duplicated pituitary fossae and cleft basisphenoid | Yes | Yes | Yes | Yes | Yes |
| Sphenopharyngeal canal | No | Yes | No | Yes | No |
| Hypertelorism | Yes | Yes | Yes | Yes | No |
| Corpus callosum | Normal | Agenesis | Agenesis | Agenesis | Normal |
| Mouth and nasopharynx | Normal | Cleft palate, oral dermoid, frontonasal dysplasia | Jaw teratoma, syngnathia, sphenomandibular-maxillary fusion, cleft palate | Cleft palate | Oral “hairy” dermoid, posterior cleft through bony palate |
| Posterior fossa | Wide pons, prominent ventral raphe | Normal | Normal | Lower brain stem tethered anteriorly to pharynx through cleft above anterior arch of C1 with basion | Lower brain stem tethered anteriorly to pharynx through cleft above anterior arch of C1 with basion |
| Basilar artery | Duplicated, MR angiogram available | Duplicated, only spin-echo images available | Duplicated, only spin-echo images available | Duplicated, MR angiogram available | Duplicated, MR angiogram available |
| Cervical spine anomalies/anomalies of basion | Yes | Yes | Yes | Yes | Yes |
Basilar Artery
Routine imaging of all patients revealed partial segmentation of the basilar artery, from the level of the midpons rostrally (Table 1). 3D time-of-flight sequence MR angiography was available for two of the five patients and confirmed duplication of the basilar artery, from the level of the midpons rostrally. The posterior cerebral artery and the superior cerebellar artery arose separately on each side from the split basilar artery. The circle of Willis was otherwise normal.
Discussion
Duplication of the pituitary gland is a rare phenomenon, with only 13 cases reported up to January 1995 and five cases reported between that time and the time of this writing (1–6). To a large extent, the formation of the hypophysis depends on interaction of the embryonic primordium with normal growth processes and materials in the prechordal region of the head. The prechordal plate and the rostral portion of the notochord are closely related to the development of the pituitary gland (7–12). Defects in the prechordal plate have been proposed as the pathogenetic mechanism of prosencephalic midline abnormalities such as holoprosencephaly and agenesis of the corpus callosum (13). Faulty interaction between the notochord, prechordal plate, and surface ectoderm may result in various craniofacial abnormalities. Inference from these early embryonic relationships suggests that duplication of the tip of the rostral end of the notochord plate or notochord may act as the primary factor that leads to duplication of the pituitary primordium, with resultant formation of two morphologically normal glands. Relevant embryologic development of the pituitary gland and pathogenesis of pituitary duplication have been reviewed in detail by Kollias et al (1).
Basilar Artery Embryology and Duplication
Branches of the internal carotid artery to the adenophypophyseal primordium develop during stage 13 (28 days of gestation) and are the earliest arteries to develop. With duplication of the hypophyseal primordium, anomalies at the circle of Willis could easily occur. Burke et al (5), in 2000, described fusiform dilation of the left A2 segment. Hori (14), in 1983, and Tagliavini and Pilleri (15), in 1986, described anomalies of the circle of Willis. However, anomalies of the basilar artery have not been reported previously in literature. In all five of our cases, distal basilar artery duplication was observed. This was well seen on routine MR images, particularly on coronal T2-weighted images. In three cases, time-of-flight MR angiograms were also available. At the 3- to 4-mm stage of the embryo, the primitive internal carotid arteries develop as a cranial extension of the paired dorsal aorta. Paired longitudinal neural arteries appear along the hindbrain, and at the 5- to 8-mm stage, they coalesce to form the basilar artery. At the 5- to 6-mm stage (29 days), the primitive posterior communicating artery forms and rostrally connects the primitive internal carotid artery with the paired precursors of the basilar artery. These persistent fetal arteries generally exist for 7 to 10 days and transiently serve as the primary blood supply to the longitudinal neural arteries. They disappear by the 14- to 15-mm stage, at which time the vertebrobasilar system is fully developed. Fusion of the embryonic longitudinal neural arteries into a single basilar artery occurs in caudocranial direction by approximately the 5th fetal week (16). As reported in literature, segmental duplication of the basilar artery can occur as a result of failure of fusion of the neural arteries and of regression of the bridging arteries that connect the longitudinal arteries (17). The word fenestration refers to a localized duplication of a vessel. Basilar artery fenestration has a reported angiographic prevalence of 0.04% to 0.6%. However, postmortem studies have reported a prevalence as high as 5.26% (18). This usually involves the lower half of the vessel, probably because of incomplete fusion of the embryonic longitudinal neural arteries, which proceeds caudocranially. By conducting a literature search, we found one case of partial duplication of the basilar artery in the distal portion of the artery in a 54-year-old man who was investigated angiographically for subarachnoid hemorrhage. In that study of 75 fixed brains and 2086 vertebral angiograms, four cases of fenestrations/duplications were found, only one of which involved the distal basilar artery (19).
In cases of pituitary duplication, we postulate that segmental duplication of the basilar artery is related to the split of the rostral end of the notochord. In our cases, the basilar arteries showed segmental duplication in their distal portions, with separate origins of the ipsilateral superior cerebellar artery and the posterior cerebral artery from each duplicated vessel. Reports presented in the literature suggest an increased risk for aneurysm formation in cases of fenestration or duplication of the basilar artery. Pathologic studies have shown that fenestrations have a structural similarity to bifurcations in major cerebral vessels. Tissue sections cut longitudinally through the fenestration have revealed an absence of the media at the edge of the fenestration similar to arterial bifurcations in the brain (20). Also at these sites, the subendothelium is aligned differently, which is probably a result of hemodynamic alterations in blood flow (21). The media defect might be embryologically ascribed to the persistence of the morphologic individuality of the tunica media of the two branches at the point at which the fusion of the primitive longitudinal neural arteries stopped. We postulate that similar hemodynamic alterations with increased risk of aneurysm formation have the potential of occurring in our cases, which could justify further surveillance. A case report of segmental duplication of the basilar artery with thrombosis in a 43-year-old man sites hemodynamic disturbances and turbulent flow as the possible cause of thrombosis (22). In the excellent review of pituitary duplications presented by Kollias et al (1), 12 of 13 patients died immediately after birth. Of our five patients, four are surviving. Hence, we think that the finding of basilar artery duplications associated with pituitary duplications is relevant.
References
- 1.Kollias SS, Ball WS, Prenger EC. Review of the embryologic development of the pituitary gland and report of a case of hypophyseal duplication detected by MRI.Neuroradiology 1995;37:3–12 [DOI] [PubMed] [Google Scholar]
- 2.Shah S, Pereira JK, Becker CJ, Roubal SE. Duplication of pituitary gland.J Comput Assist Tomogr 1997;21:459–561 [DOI] [PubMed] [Google Scholar]
- 3.Hamon-Kerautret M, Ares GS, Demondion X, Rouland V, Francke JP, Pruvo JP. Duplication of the pituitary gland in a newborn with median cleft face syndrome and nasal teratoma.Pediatr Radiol 1998;28:290–292 [DOI] [PubMed] [Google Scholar]
- 4.Lam WW, Metreweli C. MR of double hypophysis.Clin Radiol 1999;54:774–775 [DOI] [PubMed] [Google Scholar]
- 5.Burke M, Zinkovsky S, Abrantes MA, Riley W. Duplication of the hypophysis.Pediatr Neurosurg 2000;33:95–99 [DOI] [PubMed] [Google Scholar]
- 6.Vandenhaute B, Leteurtre E, Lecombe-Houcke M, Pellerin P, Nuyts JP, Cuisset JM, Soto-Ares G. Epignathus teratoma: report of three cases with a review of literature.Cleft Palate Craniofac J 2000;37:83–91 [DOI] [PubMed] [Google Scholar]
- 7.Gilbert MS. Some factors influencing the early development of mammalian hypophysis.Anat Rec 1935;62:337–359 [Google Scholar]
- 8.Muller F, O’Rahilly R. The development of the human brain from a closed neural tube at stage 13.Anat Embryol (Berl) 1988;177:203–224 [DOI] [PubMed] [Google Scholar]
- 9.Muller F, O’Rahilly R. The human brain at stage 16 including the initial evagination of the neurohypophysis.Anat Embryol (Berl) 1989;179:551–569 [DOI] [PubMed] [Google Scholar]
- 10.Ikeda H, Suzuki J, Sasano N, Niizuma H. The development and morphogenesis of the human pituitary gland.Anat Embryol (Berl) 1988;178:327–336 [DOI] [PubMed] [Google Scholar]
- 11.Bagherian V, Graham M, Gerson LP, Armstrong DL. Double pituitary glands with partial duplication of facial and fore brain structures with hydrocephalus.Comput Radiol 1984;8:203–210 [DOI] [PubMed] [Google Scholar]
- 12.O’Rahilly R, Muller F. The first appearance of the human nervous system at stage 8.Anat Embryol (Berl) 1981;163:1–13 [DOI] [PubMed] [Google Scholar]
- 13.Jellinger K, Gross H, Kaltenback E, Grisold W. Holoprosencephaly and agenesis of the corpus callosum: frequency of associated malformations.Acta Neuropathol (Berl) 1981;55:1–10 [DOI] [PubMed] [Google Scholar]
- 14.Hori A. A brain with two hypophyses in median cleft syndrome.Acta Neuropathol (Berl) 1983;59:150–154 [DOI] [PubMed] [Google Scholar]
- 15.Tagliavini F, Pilleri G. Mamillo-hypophyseal duplication (diplo-mamillo-hypophysis).Acta Neuropathol (Berl) 1986;69:38–44 [DOI] [PubMed] [Google Scholar]
- 16.Goldstein JH, Woodcock R, Do HM, Phillips CD, Dion JE. Complete duplication or extreme fenestration of the basilar artery.AJNR Am J Neuroradiol 1999;20:149–150 [PubMed] [Google Scholar]
- 17.Sanders WP, Sorek PA, Mehta BA. Fenestration of intracranial arteries with special attention to associated aneurysms and other anomalies.AJNR Am J Neuroradiol 1993;14:675–680 [PMC free article] [PubMed] [Google Scholar]
- 18.Wollschlaeger G, Wollschlaeger PB, Lucas FV, Lopez VF. Experience and result with postmortem cerebral angiography performed as routine procedure of the autopsy.Am J Roentgenol Radium Ther Nucl Med 1967;101:68–87 [DOI] [PubMed] [Google Scholar]
- 19.Trah-Dinh HD, Soo YS, Jayasinghe LS. Duplication of the vertebro-basilar system.Australas Radiol 1991;35:220–224 [DOI] [PubMed] [Google Scholar]
- 20.De Caro R, Serafini MT, Galli S, Parenti A, Guidolin D, Munari PF. Anatomy of segmental duplication in the human basilar artery: possible site of aneurysm formation.Clin Neuropathol 1995;13:303–309 [PubMed] [Google Scholar]
- 21.Finlay HM, Canham PB. The layered fabric of cerebral artery fenestrations.Stroke 1994;25:1799–1806 [DOI] [PubMed] [Google Scholar]
- 22.Berry AD III, Kepes JJ, Wetzel MD. Segmental duplication of the basilar artery with thrombosis.Stroke 1988;19:256–260 [DOI] [PubMed] [Google Scholar]