Abstract
BACKGROUND AND PURPOSE: Diffusion tensor imaging measures the proton diffusivity and preferential orientation of the diffusion tensor. X-linked adrenoleukodystrophy is a demyelinating disease for which therapy depends on the onset and extension of demyelination. We investigated the ability of diffusion tensor imaging to detect changes in the demyelinated lesions and in the normal appearing white matter.
METHODS: Diffusion tensor imaging of three related boys with X-linked adrenoleukodystrophy and seven age-matched control participants was performed. Isotropic diffusion (D′) and fractional anisotropy (FA) values were determined in 18 regions of interest in the white matter of both hemispheres.
RESULTS: In all the demyelinated white matter areas, a pattern with increased D′ and loss of FA was found. For example, mean D′ was 1.772 × 10−3mm2/s in patient 2 with blindness and extensive demyelination of the occipital white matter and was 0.693 × 10−3mm2/s in control participants (P = .01). In the same region, mean FA was 0.103 (0.464 in control participants, P < .0001). Significant alterations of D′ and FA were also observed in normal appearing white matter. For example, mean D′ was 0.802 × 10−3mm2/s in the parietal white matter of patient 1 with no visible alterations on T2-weighted images (0.715 × 10−3 mm2/s in control patients, P = .03), whereas mean FA was 0.320 (0.400 in control participants, P = .003).
CONCLUSION: Elevated D′ and loss of FA revealed by diffusion tensor imaging are consistent with severe demyelination in patients with X-linked adrenoleukodystrophy. Significant alterations of D′ and FA in normal appearing white matter may indicate early demyelination in areas that are not yet visibly altered on conventional MR images. Further evaluation in a larger series of patients and long-term study are needed.
X-linked adrenoleukodystrophy is a rare hereditary peroxisomal disorder that causes malfunction of the myelin in the CNS and of the adrenal cortex (1). The childhood cerebral form is the most frequent form. Routine MR imaging is used to calculate a severity score, as devised by Loes et al (2), based on the detection of symmetrical demyelinating lesions that occur characteristically in the parietooccipital cerebral hemispheres. The only effective treatment is bone marrow transplantation. The decision depends on the onset and extension of demyelination (3). The early visualization of demyelination plays a central role in the decision process to undertake a bone marrow transplantation that should be performed before neurologic deficits become evident.
MR diffusion tensor imaging is a noninvasive method for evaluating diffusivity and directionality of free water molecules. In white matter, diffusion is dependent on the orientation of the white matter tracks and on the degree and integrity of myelination. Measurements are being made in at least six noncolinear directions of space, but a more uniform sampling along a higher number of directions avoids sampling direction bias and increases the accuracy of diffusion tensor quantification (4). It provides an orientationally averaged measure of water diffusion (mean diffusivity, D′) and the degree of diffusion anisotropy (fractional anisotropy, FA). It has been shown that this in vivo technique is highly sensitive to variable microstructural changes of the cerebral tissue. Examples include multiple sclerosis (5, 6), Alzheimer disease (7), cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (8), and adrenoleukodystrophy (9). It has been suggested that a drop in FA reflects disintegration of the myelin sheath and axonal disruption, whereas a rise in D′ results from increased free water content and injury of structures that restrict water diffusion (6, 10). Unlike the results reported by Ito et al (9) that only D′ and FA alterations in the already demyelinated areas could be found, we compared the values of D′ and FA in the brain white matter of three boys with adrenoleukodystrophy with those of seven age-matched control participants, testing the hypothesis that diffusion tensor imaging might be able to show only early alteration of the white matter structure before it becomes visible on conventional T1- or T2-weighted images when the values are compared with those of a normal population.
Methods
Three patients of the same kindred (ages, 9, 11, and 13 years) with biochemically proved X-linked adrenoleukodystrophy were evaluated with conventional MR imaging and diffusion tensor imaging. All three patients had Addison’s disease. One patient (patient 2) was blind, and the two other patients had no neurologic deficits and attended regular school classes (patients 1 and 3). Seven age-matched neurologically normal children who underwent MR imaging were also evaluated with diffusion tensor imaging after informed consent was obtained.
MR imaging measurements were obtained at 2 T with a 30 mT/m gradient system. Diffusion-weighted imaging measurements were obtained with a multi-section single shot diffusion-weighted spin-echo echo-planar imaging sequence: 5.9 s/102 ms (TR/TE); field of view, 256 × 256 mm2; whole brain coverage; 6-mm contiguous sections. Raw data size was 128(R) × 68(Ph, 70% k-space covering). Raw data were corrected for sampling errors (regridding) and were reconstructed to a 128 × 128 image size. Diffusion encoding was performed along 60 directions evenly distributed over a sphere. Diffusion weighting was optimized according to the method presented by Jones et al (4) (ie, only one measurement with a high diffusion weighting was obtained for each of 60 diffusion-encoding gradient directions and six measurements were obtained with a low diffusion weighting). The “effective b factor” (sum of diagonal elements of the b-factor matrix) of approximately 1500 s/mm2 was chosen to be close to the optimum value of 1/D′ in terms of signal intensity-to-noise ratio (4, 11). The total measurement time was approximately 6.5 min. The images were corrected for eddy current-induced geometrical distortions (magnification, translation, and shear) according to a modified procedure presented by Bastin and Armitage (12). The b matrix was calculated numerically, including all imaging and diffusion-encoding gradients. Diffusion tensor components were computed pixel by pixel and were diagonalized to find the fiber direction by using homemade routines developed on Matlab (MathWorks, Inc.).
All the generated maps were reviewed by two authors (JFLS and EM). D′ and FA values were obtained in both hemispheres after placing manually selected regions of interest in the following white matter areas: corticospinal tracts (in the centrum semiovale), posterior limb of the internal capsule, anterior limb of the internal capsule and parietal white matter, occipital white matter, temporal white matter, and frontal white matter, genu corporis callosi, and splenium corporis callosi. The regions of interest were placed in anatomic localization, respecting the shape of the deep white matter areas, which precludes the use of predefined regions of interest. The sizes of the regions of interest varied among patients and control participants (see Tables 1 and 2 for the exact region of interest volume expressed in pixels).
TABLE 1:
Mean diffusivity (D′ × 10−3 mm2/s)
| D′ | Patient 1 |
Patient 2 |
Patient 3 |
Control Participants |
Mean +1 SD | Mean +2 SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | ROI | T2 | Mean | SD | ROI | T2 | Mean | SD | ROI | T2 | Mean | SD | |||
| PLIC-l | 0.65 | 0.02 | 9 | 0 | 0.70 | 0.08 | 9 | 1 | 0.70 | 0.05 | 9 | 1 | 0.65 | 0.02 | 0.67 | 0.68 |
| PLIC-r | 0.67 | 0.03 | 8 | 0 | 0.69 | 0.06 | 9 | 1 | 0.68 | 0.06 | 9 | 1 | ||||
| CSO-l | 0.66 | 0.03 | 29 | 0 | 0.78 | 0.08 | 36 | 1 | 0.70 | 0.03 | 51 | 0 | 0.66 | 0.02 | 0.68 | 0.70 |
| CSO-r | 0.66 | 0.02 | 25 | 0 | 0.77 | 0.10 | 38 | 1 | 0.71 | 0.03 | 52 | 0 | ||||
| FWM-l | 0.77 | 0.04 | 62 | 0 | 0.74 | 0.04 | 80 | 0 | 0.75 | 0.05 | 100 | 0 | 0.74 | 0.02 | 0.76 | 0.78 |
| FWM-r | 0.77 | 0.04 | 80 | 0 | 0.74 | 0.05 | 103 | 0 | 0.73 | 0.06 | 105 | 0 | ||||
| PWM-l | 0.79 | 0.08 | 85 | 0 | 1.10 | 0.36 | 160 | 1 | 0.66 | 0.09 | 112 | 0 | 0.71 | 0.02 | 0.73 | 0.76 |
| PWM-r | 0.82 | 0.08 | 75 | 0 | 1.08 | 0.42 | 206 | 1 | 0.71 | 0.07 | 158 | 0 | ||||
| TWM-l | 0.82 | 0.08 | 93 | 0 | 1.13 | 0.30 | 91 | 1 | 0.81 | 0.08 | 100 | 0 | 0.77 | 0.02 | 0.79 | 0.80 |
| TWM-r | 0.81 | 0.08 | 113 | 0 | 1.32 | 0.36 | 94 | 1 | 0.79 | 0.10 | 130 | 0 | ||||
| OWM-l | 0.81 | 0.15 | 90 | 0 | 1.82 | 0.54 | 77 | 1 | 0.72 | 0.21 | 69 | 0 | 0.70 | 0.04 | 0.73 | 0.77 |
| OWM-r | 0.78 | 0.17 | 71 | 0 | 1.72 | 0.53 | 85 | 1 | 0.71 | 0.12 | 96 | 0 | ||||
| GCC-l | 0.84 | 0.30 | 24 | 0 | 0.81 | 0.12 | 22 | 0 | 0.72 | 0.20 | 21 | 0 | 0.81 | 0.09 | 0.90 | 1.00 |
| GCC-r | 0.83 | 0.20 | 18 | 0 | 0.80 | 0.16 | 20 | 0 | 0.68 | 0.09 | 26 | 0 | ||||
| SCC-l | 0.78 | 0.18 | 28 | 0 | 1.92 | 0.35 | 16 | 1 | 0.63 | 0.25 | 27 | 0 | 0.73 | 0.06 | 0.78 | 0.84 |
| SCC-r | 0.79 | 0.13 | 20 | 0 | 2.13 | 0.38 | 18 | 1 | 0.61 | 0.10 | 19 | 0 | ||||
| ALIC-l | 0.72 | 0.06 | 11 | 0 | 0.77 | 0.04 | 13 | 0 | 0.86 | 0.10 | 16 | 1 | 0.73 | 0.05 | 0.79 | 0.84 |
| ALIC-r | 0.75 | 0.09 | 9 | 0 | 0.73 | 0.06 | 8 | 0 | 0.82 | 0.11 | 12 | 1 | ||||
Note.—D′ indicates isotropic diffusion; ROI, region of interest; T2, T2 value on T2-weighted MR images; -l, on the left side; -r, on the right side; PLIC, posterior limb of the internal capsule; CSO, centrum semiovale; FWM, frontal white matter; PWM, parietal white matter; TWM, temporal white matter; OWM, occipital white matter; GCC, genu corporis callosi; SCC, splenium corporis callosi; ALIC, anterior limb of the internal capsule. Mean diffusivity values for three patients with X-linked adrenoleukodystrophy, indicated as mean values and intra-region of interest SDs (inter-pixel SDs), are compared with those of seven age-matched normal control participants, indicated as mean values and inter-region of interest SDs. Values shown on light gray background are between 1 and 2 SDs of the mean, and values shown on dark gray background are >2 SDs of the mean, compared with values for normal control participants. T2 values are either 0 for T2-weighted images without evidence of demyelination in the specific region or 1 for T2-weighted images with evidence of demyelination.
TABLE 2:
Fractional anisotropy index
| FA | Patient 1 |
Patient 2 |
Patient 3 |
Control Participant |
Mean −1 SD | Mean −2 SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | ROI | T2 | Mean | SD | ROI | T2 | Mean | SD | ROI | T2 | Mean | SD | |||
| PLIC-l | 0.76 | 0.05 | 9 | 0 | 0.68 | 0.06 | 9 | 1 | 0.73 | 0.10 | 9 | 1 | 0.76 | 0.02 | 0.75 | 0.73 |
| PLIC-r | 0.72 | 0.06 | 8 | 0 | 0.65 | 0.06 | 9 | 1 | 0.75 | 0.04 | 9 | 1 | ||||
| CSO-l | 0.56 | 0.08 | 29 | 0 | 0.32 | 0.11 | 36 | 1 | 0.50 | 0.06 | 51 | 0 | 0.54 | 0.05 | 0.48 | 0.43 |
| CSO-r | 0.57 | 0.07 | 25 | 0 | 0.32 | 0.13 | 38 | 1 | 0.48 | 0.07 | 52 | 0 | ||||
| FWM-l | 0.36 | 0.10 | 62 | 0 | 0.37 | 0.10 | 80 | 0 | 0.33 | 0.09 | 100 | 0 | 0.38 | 0.04 | 0.34 | 0.31 |
| FWM-r | 0.37 | 0.10 | 80 | 0 | 0.38 | 0.09 | 103 | 0 | 0.38 | 0.11 | 105 | 0 | ||||
| PWM-l | 0.33 | 0.09 | 85 | 0 | 0.11 | 0.06 | 160 | 1 | 0.39 | 0.07 | 112 | 0 | 0.40 | 0.02 | 0.38 | 0.35 |
| PWM-r | 0.31 | 0.11 | 75 | 0 | 0.11 | 0.05 | 206 | 1 | 0.40 | 0.08 | 158 | 0 | ||||
| TWM-l | 0.44 | 0.08 | 93 | 0 | 0.13 | 0.04 | 91 | 1 | 0.40 | 0.07 | 100 | 0 | 0.47 | 0.03 | 0.43 | 0.40 |
| TWM-r | 0.42 | 0.10 | 113 | 0 | 0.12 | 0.03 | 94 | 1 | 0.41 | 0.08 | 130 | 0 | ||||
| OWM-l | 0.47 | 0.10 | 90 | 0 | 0.11 | 0.07 | 77 | 1 | 0.46 | 0.10 | 69 | 0 | 0.46 | 0.04 | 0.42 | 0.38 |
| OWM-r | 0.44 | 0.11 | 71 | 0 | 0.10 | 0.05 | 85 | 1 | 0.44 | 0.13 | 96 | 0 | ||||
| GCC-l | 0.66 | 0.13 | 24 | 0 | 0.68 | 0.12 | 22 | 0 | 0.69 | 0.12 | 21 | 0 | 0.72 | 0.07 | 0.66 | 0.59 |
| GCC-r | 0.75 | 0.10 | 18 | 0 | 0.64 | 0.13 | 20 | 0 | 0.67 | 0.10 | 26 | 0 | ||||
| SCC-l | 0.72 | 0.10 | 28 | 0 | 0.21 | 0.03 | 16 | 1 | 0.79 | 0.15 | 27 | 0 | 0.78 | 0.05 | 0.73 | 0.68 |
| SCC-r | 0.76 | 0.06 | 20 | 0 | 0.20 | 0.02 | 18 | 1 | 0.81 | 0.08 | 19 | 0 | ||||
| ALIC-l | 0.53 | 0.13 | 11 | 0 | 0.49 | 0.09 | 13 | 0 | 0.38 | 0.06 | 16 | 1 | 0.54 | 0.06 | 0.48 | 0.43 |
| ALIC-r | 0.62 | 0.08 | 9 | 0 | 0.60 | 0.12 | 8 | 0 | 0.43 | 0.04 | 12 | 1 | ||||
Note.—FA indicates fractional anisotropy; ROI, region of interest; T2, T2 value on T2-weighted MR images; -l, on the left side; -r, on the right side; PLIC, posterior limb of the internal capsule; CSO, centrum semiovale; FWM, frontal white matter; PWM, parietal white matter; TWM, temporal white matter; OWM, occipital white matter; GCC, genu corporis callosi; SCC, splenium corporis callosi; ALIC, anterior limb of the internal capsule. Fractional anisotropy values for three patients with X-linked adrenoleukodystrophy, indicated as mean values and intra-region of interest SDs (inter-pixel SDs), are compared with those of seven age-matched normal control participants, indicated as mean values and inter-region of interest SDs. Values shown on light gray background are between 1 and 2 SDs of the mean, and values shown on dark gray background are >2 SDs of the mean, compared with values for normal control participants. T2 values are either 0 for T2-weighted images without evidence of demyelination in the specific region or 1 for T2-weighted images with evidence of demyelination.
All three patients underwent conventional native and contrast-enhanced T1- and T2-weighted imaging. White matter was classified as normal appearing white matter or white matter with increased signal intensity on T2-weighted images. The averages and SDs of the measured areas were calculated and compared with those of seven age-matched control participants who were referred for routine neuroradiologic examinations, who had no neurologic deficits (excluding mental retardation, chronic seizures), and for whom no pathologic abnormality was detected based on MR imaging of the brain.
Results
The T2-weighted images of patient 1 had no signal intensity alteration. The T2-weighted images of patient 2, who was blind, had extensive confluent and symmetric signal intensity abnormalities involving multiple white matter areas: parietal white matter, temporal white matter, occipital white matter, splenium corporis callosi, posterior limb of internal capsule, and centrum semiovale. The T2-weighted images of patient 3 had mild signal intensity alterations in the anterior limb of the internal capsule and, to a lesser extent, in the posterior limb of the internal capsule. The images of patients 1 and 3 showed no pathologic contrast enhancement, whereas the images of patient 2 showed typical rim enhancement at the periphery of the demyelination area.
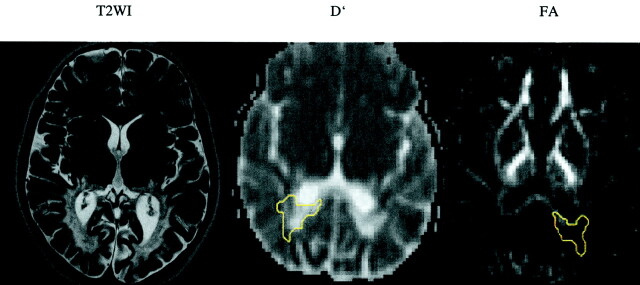
In all the T2-hyperintense regions, a significant increase, defined as a value of >2 SDs from the mean of the control values, in D′ (Table 1) and a decrease in FA (Table 2) were noted. The values showed the greatest changes in the center of the extensive demyelinated areas, with D′ as high as 1.82 × 10−3mm2/s in the occipital white matter and 1.92 × 10−3mm2/s in the splenium corporis callosi and with FA as low as 0.10 in the occipital white matter and 0.20 in the splenium corporis callosi of patient 2 (Fig 1).
Fig 1.
T2-weighed image (T2WI) and D′ and FA maps of the blind patient (patient 2) shows confluent and symmetric abnormalities in the deep white matter of both occipital lobes. Regions of interest are shown on D′ (−r) and FA (−l) maps (yellow outlines).
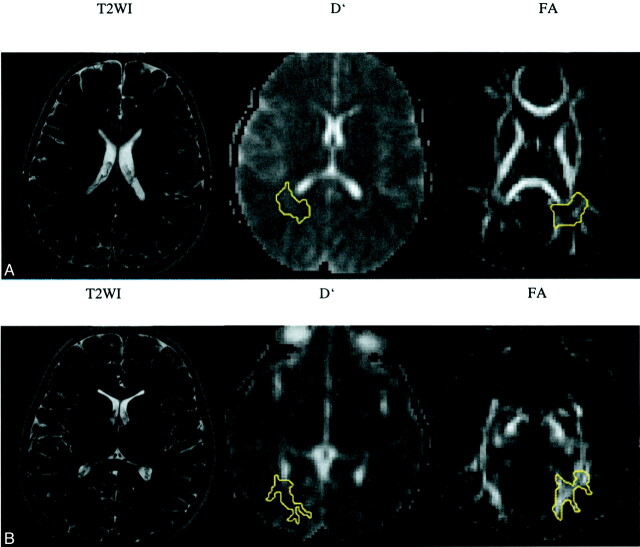
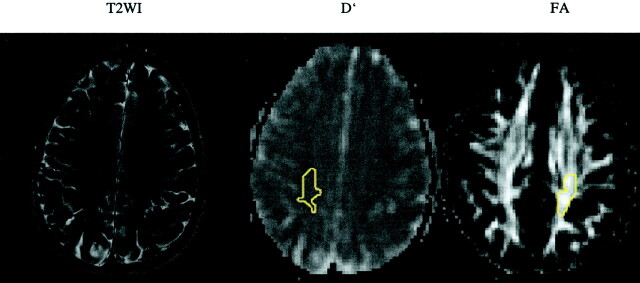
The areas of deep white matter that showed no abnormality on the T2-weighted images of patient 2, who had the most extensive demyelinating lesions, also had D′ and FA values that were within normal range. In the other two patients (patient 1, no visible abnormalities; patient 3, minimal abnormalities), significant D′ changes were documented. For patient 1, the changes were seen in parietal white matter, temporal white matter, and occipital white matter, with D′ values falling short of 2 SDs in frontal white matter and splenium corporis callosi; significant FA changes were also registered in the parietal white matter, with FA values falling short of 2 SDs in temporal white matter on the right, genu corporis callosi on the left, and splenium corporis callosi on the left (Fig 2). For patient 3, significant D′ changes were documented as occurring in centrum semiovale and temporal white matter; FA changes with values falling short of 2 SDs were documented in temporal white matter (Fig 3). The general trend for these two patients (patients 1 and 3) was that D′ values showed a pathologic change that was more pronounced than that of FA values in the chosen normal appearing white matter areas, whereas the reverse did not occur.
Fig 2.
T2-weighted images (T2WI) and D′ and FA maps of patient 1.
A, No signal intensity alteration is shown in the parietal white matter.
B, No signal intensity alteration is shown in the occipital deep white matter. Regions of interest are shown on D′ (−r) and FA (−l) maps (yellow outlines).
Fig 3.
T2-weighted image (T2WI) and D′ and FA maps of patient 3. No signal intensity alteration is shown in the centrum semiovale. Regions of interest are shown on D′ (−r) and FA (−l) maps (yellow outlines).
Discussion
The diffusion tensor study was undertaken to investigate the tissue microstructure of the white matter in patients with X-linked adrenoleukodystrophy. Bone marrow transplantation, as the only efficient treatment of this disease, is based on clinical and radiologic evidence of the presence and extension of demyelination. The degree of demyelination is based on signal intensity alteration in T2-weighted images, the presence of atrophy, and the presence of contrast enhancement at the boundaries of the demyelinating zones. This is summed in a severity score devised by Loes et al (2). Bone marrow transplantation has a favorable effect in boys and male adolescents who show early evidence of cerebral involvement. It is not indicated for patients who do not have evidence of brain involvement, for those who have advanced brain involvement, or for those who have adrenomyeloneuropathy. Baseline Loes scores of ≥3 correlate with poorer prognosis. Normal MR imaging findings constitute a favorable sign for patients who are older than 7 years, but that is not true for younger children (1). Identifying early demyelination might have an implication on the exact timing of bone marrow transplantation because there is only a limited time window during which bone marrow transplantation is to be considered, even if no consensus has been reached regarding when exactly this procedure should be undertaken. A first step toward identifying early demyelination has been made by using spectroscopic imaging with which alterations in normal appearing white matter were shown to correlate with progressive disease (13). Our measurements were optimized for accuracy in diffusion parameter estimation within the limited time available for clinical examination. The monoexponential diffusion decay was intrinsically assumed, and therefore, only a single high b value along each direction was measured. Recent studies with b values as high as 6000 s/mm2 have shown a biexponential apparent diffusion coefficient decay (14–16). Such deviation from the monoexponential model could cause systematic error in diffusion parameter estimation, which is b value dependent. However, according to the studies noted above, such errors become significant with a b factor approaching 3000 s/mm2. With a b factor of ≤1500 s/mm2, the variations in diffusion parameters are small and almost negligible.
Our results show a significant (defined as value of >2 SD from the mean of the control group) increase in D′ and a decrease in FA within affected areas. That suggests that there is an increase in the free water fraction and injury of the white matter microstructures that restrict water diffusion (high D′) and destruction of myelin sheath and loss of the axons’ integrity (reduced FA), correlating well with previously published data (9).
However, a normative database is of special importance in childhood because D′ and FA values are known to vary with the state of myelination whereas the most extensive changes take place during the first 2 years of life (17, 18). Comparison with normative values allowed us to show that these indices are altered in normal appearing white matter in two of three patients, suggesting subtle alterations of the microstructural state of white matter tracts. These changes may precede signal intensity alterations as seen on conventional images, on which more macrostructural lesions are present. Further analysis of the results revealed that in the normal appearing white matter areas, where D′ and FA were altered, the alteration of D′ showed a more pronounced alteration than the alteration of FA. An explanation for this trend might be that during early demyelination, when there is a myelin breakdown but no inflammatory cell component, thus corresponding to the first zone ad reported by Schaumberg et al (19), extracellular water accumulates and mobility of the extracellular water molecules increases in the edematous interstitium, leading to a higher D′ value. During a second phase, because of the pronounced inflammatory process, myelin breakdown is accompanied by a beginning loss of axonal integrity that correlates with a decline in the FA value. During a last phase, corresponding to the core of the lesions, total liquefaction of the structures occurs, leading to extreme high D′ and low FA, approaching water values. With this hypothesis, alteration of D′ values would be the most sensitive and the earliest parameter (with alterations of FA being the next most sensitive and next earliest parameter) that could detect the beginning of demyelination in cases of X-linked adrenoleukodystrophy. The very small number of patients and the lack of follow-up make the findings of this study very preliminary, with statistical analysis being at the limit of significance. The following point, in particular, should be noted: SDs within the anatomically placed regions of interest in our control participants were lower than SDs within the regions of interest in our three patients in those locations that were suspected to be altered. On the other hand, SDs between anatomically placed regions of interest in our control participants was low, reflecting the homogeneity of the control population and the reliability of our method. This might indicate that the patient’s white matter is inhomogeneously altered, leading to a higher variation of D′ and FA inside the regions of interest. These results need to be tested on a larger population, and follow-up studies are mandatory to prove that diffusion tensor imaging alterations are forerunners of T2-weighted imaging alterations.
Conclusion
Diffusion tensor imaging allowed precise quantification of D′ and FA values in control participants and patients with X-linked adrenoleukodystrophy. Deeply altered D′ and FA are characteristic of the center of the demyelinated lesions, where myelin breakdown is most advanced and axonal structure is disintegrated. A significant increase in D′ and a decrease in FA in normal appearing white matter, suggesting an early demyelinating process, were nevertheless observed when the values were compared with those of normal age-matched control participants. The MR severity score presented by Loes et al (2) might therefore underestimate the severity of white matter injury, and therapeutic decisions should include diffusion tensor imaging in the decision process as an early indicator of demyelination. This punctual study of only three patients needs validation through larger and longitudinal studies to verify not only the results reported herein but also that altered normal appearing white matter on diffusion tensor images will eventually become altered on T2-weighted images.
References
- 1.Moser HW, Loes DJ, Melhem ER, et al. X-linked adrenoleukodystrophy: overview and prognosis as a function of age and brain magnetic resonance imaging abnormality: a study involving 372 patients. Neuropediatrics 2000;31:227–239 [DOI] [PubMed] [Google Scholar]
- 2.Loes DJ, Hite S, Moser H, et al. Adrenoleukodystrophy: a scoring method for brain MR observations. AJNR Am J Neuroradiol 1994;15:1761–1766 [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki Y, Isogai K, Teramoto T, et al. Bone marrow transplantation for the treatment of X-linked adrenoleukodystrophy. J Inherit Metab Dis 2000;23:453–458 [DOI] [PubMed] [Google Scholar]
- 4.Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 1999;42:515–525 [PubMed] [Google Scholar]
- 5.Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology 1999;52:1626–1632 [DOI] [PubMed] [Google Scholar]
- 6.Tievsky AL, Ptak T, Farkas J. Investigation of apparent diffusion coefficient and diffusion tensor anisotrophy in acute and chronic multiple sclerosis lesions. AJNR Am J Neuroradiol 1999;20:1491–1499 [PMC free article] [PubMed] [Google Scholar]
- 7.Hanyu H, Shindo H, Kakizaki D, Abe K, Iwamoto T, Takasaki M. Increased water diffusion in cerebral white matter in Alzheimer’s disease. Gerontology 1997;43:343–351 [DOI] [PubMed] [Google Scholar]
- 8.Molko N, Pappata S, Mangin JF, et al. Diffusion tensor imaging study of subcortical gray matter in cadasil. Stroke 2001;32:2049–2054 [DOI] [PubMed] [Google Scholar]
- 9.Ito R, Melhem ER, Mori S, Eichler FS, Raymond GV, Moser HW. Diffusion tensor brain MR imaging in X-linked cerebral adrenoleukodystrophy. Neurology 2001;56:544–547 [DOI] [PubMed] [Google Scholar]
- 10.Neil JJ, Shiran SI, McKinstry RC, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology 1998;209:57–66 [DOI] [PubMed] [Google Scholar]
- 11.Conturo TE, McKinstry RC, Aronovitz JA, Neil JJ. Diffusion MRI: precision, accuracy and flow effects. NMR Biomed 1995;8:307–332 [DOI] [PubMed] [Google Scholar]
- 12.Bastin ME, Armitage PA. On the use of water phantom images to calibrate and correct eddy current induced artefacts in MR diffusion tensor imaging. Magn Reson Imaging 2000;18:681–687 [DOI] [PubMed] [Google Scholar]
- 13.Eichler FS, Barker PB, Cox C, et al. Proton MR spectroscopic imaging predicts lesion progression on MRI in X-linked adrenoleukodystrophy. Neurology 2002;58:901–907 [DOI] [PubMed] [Google Scholar]
- 14.Mulkern RV, Vajapeyam S, Robertson RL, Caruso PA, Rivkin MJ, Maier SE. Biexponential apparent diffusion coefficient parametrization in adult vs newborn brain. Magn Reson Imaging 2001;19:659–668 [DOI] [PubMed] [Google Scholar]
- 15.Maier SE, Bogner P, Bajzik G, et al. Normal brain and brain tumor: multicomponent apparent diffusion coefficient line scan imaging. Radiology 2001;219:842–849 [DOI] [PubMed] [Google Scholar]
- 16.Clark CA, Le Bihan D. Water diffusion compartmentation and anisotropy at high b values in the human brain. Magn Reson Med 2000;44:852–859 [DOI] [PubMed] [Google Scholar]
- 17.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology 2002;222:212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee P, Miller JH, Shimony JS, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology 2001;221:349–358 [DOI] [PubMed] [Google Scholar]
- 19.Schaumburg HH, Powers JM, Raine CS, Suzuki K, Richardson EP Jr. Adrenoleukodystrophy: a clinical and pathological study of 17 cases. Arch Neurol 1975;32:577–591 [DOI] [PubMed] [Google Scholar]