Abstract
BACKGROUND AND PURPOSE: Periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER or PROP) is an effective means of compensating for head motion during MR imaging in adults. The aim of this study was to assess the value of this novel technique in unsedated children.
METHODS: PROP T2-weighted fast spin-echo (FSE) imaging (TR/TE/NEX, 4000/83/2; 50 seconds) and T2-weighted single-shot FSE (SS-FSE) imaging (19,929/92/0.5; imaging time, 25 seconds) were performed in 35 unsedated children (mean age, 4.7 years ± 4.2) who were undergoing brain MR imaging. Two observers assessed unlabelled images for motion artifact, other artifacts, visibility of pathology, and the preferred image overall. Sequences were compared by using the χ2 test and concordant data from both observers.
RESULTS: Both PROP and the SS-FSE imaging offered equal degrees of motion correction. Metallic artifacts were worse on PROP imaging, likely because of a higher receiver bandwidth (P < .001, χ2 test). Pathology was present in 28 subjects and equally well seen on PROP and SS-FSE images. Overall, PROP was preferred, largely because of its improvements in image contrast (P < .001, χ2 test).
CONCLUSION: SS-FSE imaging and PROP provide equal motion correction, although PROP enables better assessment of the brain parenchyma.
The pediatric patient is particularly prone to movement during MR imaging. Therefore, sedation or general anesthesia is widely used to help in the diagnostic imaging of these patients. Alternatively, rapid imaging techniques, such as single-shot fast spin-echo (SS-FSE) can be used in the unsedated child to provide a limited assessment of the brain; for example, an evaluation of ventricular size (1–3). This sequence, however, has low contrast and resolution, and it offers a poor depiction of parenchymal details.
MR imaging with periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER or PROP) offers an alternative approach to imaging in the unsedated child (4, 5). While SS-FSE limits motion artifact by allowing imaging as quickly as possible, a unique mode of data acquisition with PROP allows it to intrinsically compensate for translational or rotational head motion during data acquisition.
The aim of this study was to compare both sequences in the unsedated child, with an evaluation of the presence of motion artifacts and image quality.
Methods
PROP MR Imaging was tested in clinical patients after approval was obtained from the local institutional review board.
Clinical Patients
We evaluated 35 consecutive unsedated children who were undergoing brain MR imaging at our institution. The children had a mean age of 4.7 years ± 4.2, with an age range of 9 days to 14 years. When the child was unable to lie still, a parent or guardian, an MR technologist, or a nurse manually immobilized the child’s head. A total of 33 subjects underwent MR imaging for a suspicion of hydrocephalus, and two underwent imaging for follow-up evaluation of extra-axial collections. Of these, 20 had a ventricular catheter in situ. Stigmata of either a current or a treated Chiari malformation were present in 12 children (Chiari I in two and Chiari II in 10). Focal regions of encephalomalacia were present in 12 subjects. Other visualized pathologies included cervical spine syrinx in one patient, a Dandy-Walker malformation in one, partial agenesis of the corpus callosum in one, gray matter heterotopia in one, and platybasia in one.
Imaging Parameters
Subjects were imaged by using a 1.5-T N/Vi echo-speed or high-speed machine (GE Medical Systems, Milwaukee, WI). They underwent SS-FSE (matrix size, 320 × 256; TR/TE/NEX, 19,929/92/0.5; 5-mm section thickness; FOV, 24 cm; approximately 25 sections; imaging time, 25 seconds). This was followed by PROP FSE (matrix size, 224 × 224; 4,000/83/2; 5-mm section thickness; FOV, 24 cm; approximately 25 sections; imaging time, 50 seconds) as part of the imaging protocol. In two subjects, SS-FSE was repeated with extra immobilization, because excessive motion had rendered the first study nondiagnostic. The nondiagnostic studies were discarded. In these subjects, PROP was performed only once.
PROP Data Collection, Motion Estimation, and Correction
PROP is a unique means of data collection and reconstruction that corrects for patient motion (4, 5). This correction is made possible with the acquisition of data with a series of concentric blades, each of which rotates through the center of k-space (Fig 1). This offers two main benefits over conventional means of data collection. First, the central region of k-space is sampled multiple times; this approach offers improved artifact suppression. Second, data within this central region can be compared between each blade. If motion has occurred between the acquisition of each blade, the data can be transposed and rotated to its estimated stationary position, before final image reconstruction.
Fig 1.
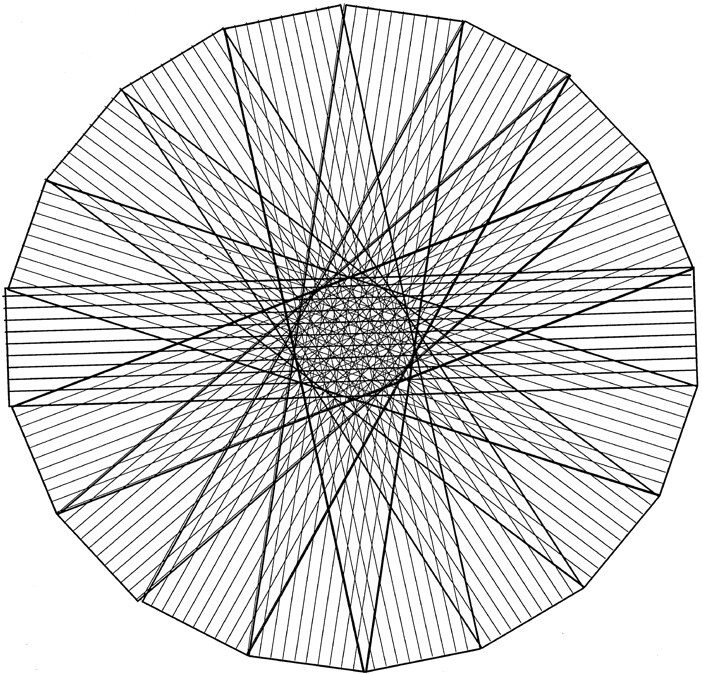
Illustration of PROP k-space data acquisition. Data are acquired in a series of rotating blades, each of which collects data from the central area of k-space. Each blade contains several phase-encode lines.
Central k-space data from the first blade was chosen as the stationary dataset and compared with corresponding data from each subsequent PROP blade. Data were correlated with the stationary dataset, first by rotating the blade, by using the data magnitude in k-space, and then by translation by using the complex data in k-space. The process was then repeated by using the average of all corrected blades as the stationary dataset, which was found to further improve image quality. Data that remained noncorrelative after in-plane translation and rotation correction were selectively weighted (6).
Image Assessment and Statistical Analysis
Two independent radiologists (J.E.M., J.P.K.) directly compared unlabelled PROP and SS-FSE images on a digital workstation (DR Systems, San Diego, CA). They examined the studies for artifacts or image blurring due to patient motion. The observers scored each study as follows: 0, free of motion; 1, mild motion; 2, moderate motion; or 3, severe motion. They also assessed for the presence of other artifacts and commented on the severity and suspected cause on both PROP and SS-FSE images. For subjects with demonstrable pathology, the observers were asked if one sequence showed the pathology best or if they were both equal. Lastly, the observers were asked to declare if they preferred a sequence or if the sequences were equal. The observers were not given strict criteria to guide these choices but were asked to give their subjective opinion. Concordant findings from the two observers were used in subsequent analysis. Interobserver variability was calculated by using the κ statistic. Significance was tested by using the χ2 test.
Results
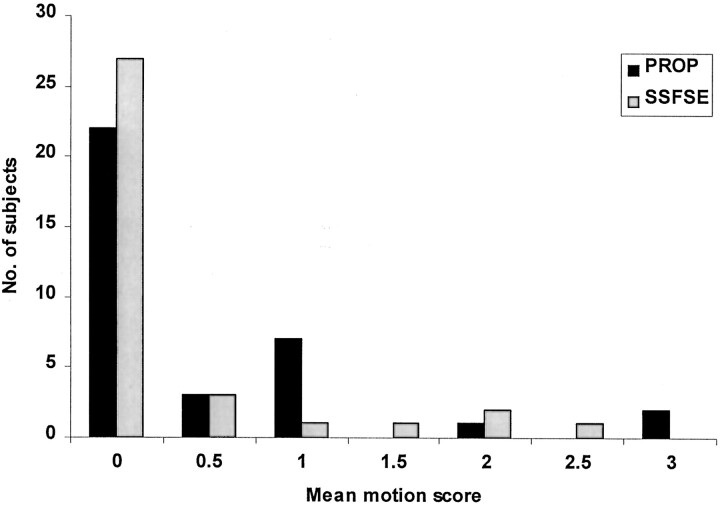
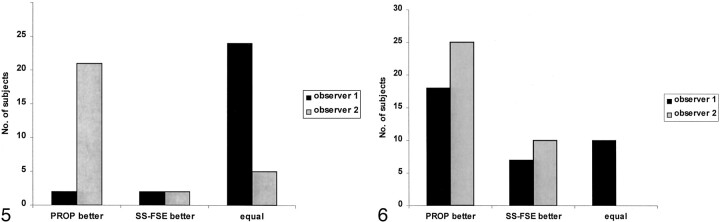
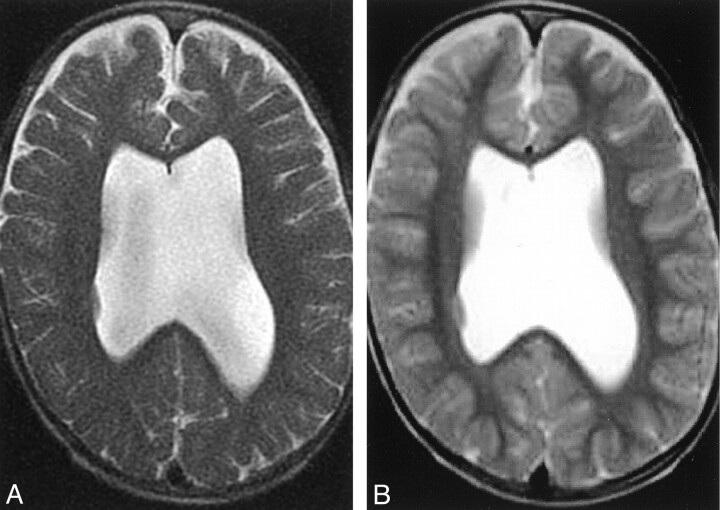
The degree of head motion was not significantly different on PROP and SS-FSE images (κ = 0.88) (Figs 2–4). In 18 subjects, other artifacts were deemed present by both observers (κ = 0.63). These artifacts arose from ventricular catheters in 16 children and from metallic clips in two (Fig 3). On the basis of the concordant results from the two observers, these artifacts were judged worse on PROP images (P < .001, χ2 test; κ = 0.60). PROP images were worse in 13 children, SS-FSE images were worse in none, both images were equal in two, and they were nonconcordant in two. Both observers agreed that pathology was present in 28 cases (κ = 0.85). Pathology was equally well seen on PROP and SS-FSE images (Fig 5). Overall, PROP was preferred (P < .001, χ2 test; κ = 0.40) (Figs 6 and 7).
Fig 2.
Bar graph shows the mean score of the two observers in grading both PROP and SS-FSE images for visible effects of motion. Scores were as follows: 0, no motion; 1, mild motion; 2, moderate motion; and 3, severe motion. On most images, no motion effects were seen, and PROP and SS-FSE performed equally in terms of motion correction.
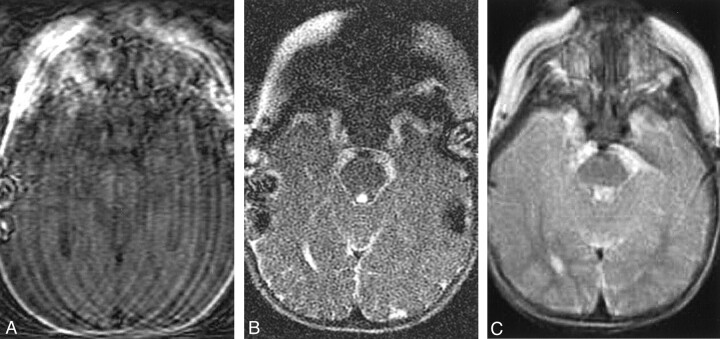
Fig 4.
Compensation of severe head motion with SS-FSE and PROP FSE imaging.
A, T1-weighted image shows the effects of severe head motion, with notable motion artifact and image blurring.
B, SS-FSE image shows a marked reduction in motion artifact, although the artifact remains even when a parent is holding the child’s head still.
C, PROP FSE image also offers a substantial reduction in motion artifact, although the image still demonstrates some blurring.
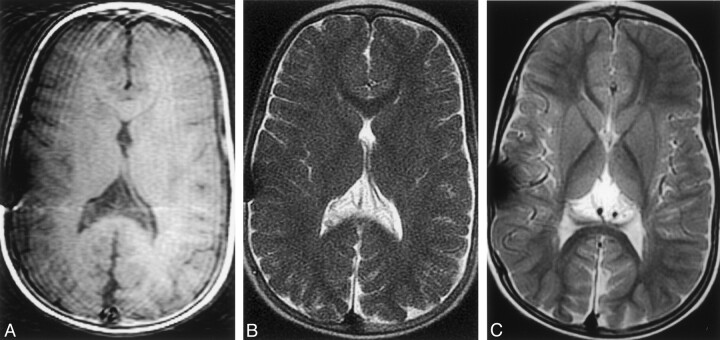
Fig 3.
Compensation of moderate head motion with SS-FSE and PROP FSE imaging.
A, T1-weighted image shows the effects of head motion. Motion artifact and variable signal intensity are demonstrated across the image.
B, SS-FSE image shows no evidence of motion artifact and enables a good assessment of ventricular size.
C, PROP FSE image offers improved gray matter-white matter differentiation. Note that artifact overlying the right side of the skull. This was caused by a ventricular catheter and was worse on this study than on others because of a higher receiver bandwidth.
Fig 5.
Visibility of pathology on SS-FSE and PROP images. Bar graph shows the impressions of the two observers regarding the images that depicted pathology most clearly. While observer 1 judged most of the images to be equal, observer 2 thought that PROP images offered an advantage.
Fig 6.
Bar graph reveals the sequences that the observers preferred. Both observers clearly preferred PROP images because of improvements in the contrast of the brain parenchyma.
Fig 7.
Improved image contrast with PROP MR imaging.
A, SS-FSE image of the brain allows assessment of ventricular size and subarachnoid spaces, but assessment of the brain parenchyma is limited.
B, PROP FSE image offers improved contrast, which allows a more-detailed assessment of both gray matter and white matter. This improvement allows the identification of a small focus of gray matter heterotopia in the lateral wall of the right lateral ventricle.
Discussion
Traditional approaches to MR imaging require a stationary subject to produce a diagnostic image. Motion occurring during data acquisition causes image artifacts, a loss of resolution, and a reduction in the signal-to-noise ratio. These effects combine to reduce anatomic detail and limit the detection of pathologic findings (7, 8). As a result, sedation or general anesthesia is required in most children undergoing routine brain MR imaging; however, these procedures pose inherent risks of adverse events such as apnea and hypoxic brain damage (9).
Recent technological advances in MR imaging now offer the possibility of producing a diagnostic image in a moving subject. Although MR imaging performed with the patient under sedation offers optimal image quality, brain imaging in unsedated patients has a growing role in the assessment of gross intracranial pathologies, such as hydrocephalus. In this situation, MR imaging now offers an alternative to CT, without the inherent risks of ionizing radiation for either the child or an adult immobilizing the child. Further, MR imaging offers substantial improvement in the contrast in the brain parenchyma.
Our findings suggest that PROP MR imaging offers a degree of motion compensation comparable to that of SS-FSE, while offering an additional improvement in image contrast. However, direct comparison of the sequences is limited by uncertainty regarding the similarity of patient motion during each of the acquisitions. For our analysis, we assumed the same degree of head motion during each. Each sequence limits the effects of motion by a different means. SS-FSE imaging offers a extremely rapid acquisition of sequential sections with imaging times of approximately 1 second per section (1–3), while PROP offers inherent motion correction (4).
PROP MR imaging is a unique means of data acquisition and reconstruction that offers a substantial advantage over conventional MR imaging because it provides a means of compensating for bulk in-plane patient motion (4, 5). As such, its motion-correction capabilities extend beyond those of projection-reconstruction (10) or spiral MR imaging (11). Unlike navigator echo sequences, all PROP data are incorporated into the image, without need for additional data collection (12).
Although the use of PROP compares favorably to SS-FSE in the unsedated child, the motion-correction capabilities of PROP fall short of what we have observed in adults (5). The likely reason is that unsedated children often have far more extreme head motion while they are within the MR unit. While PROP offers a satisfactory means of correcting for in-plane motion, through-plane motion is likely to be a notable problem in the child, and the technique does not currently corrected for this problem. Through-plane motion can be partially addressed by unevenly weighting the noncorrelative data (4).
The disadvantages of PROP compared with SS-FSE are minimal. The imaging time of PROP is slightly longer than that of SS-FSE; however, both techniques were performed in less than a minute, and this time it is unlikely to be an important factor. Susceptibility artifacts, commonly due to ventricular catheters, were more pronounced with PROP imaging than with SS-FSE imaging. This difference reflects the higher receiver bandwidth of PROP.
Some discrepancy was noted in the findings of our observers. Observer 2 more often preferring PROP for the detection of pathology, whereas observer 1 found both sequences to be equally good. A similar discrepancy was present between the two observers in declaring the preferred image. Retrospectively, observers were asked to explain their choices: Observer 2 believed that his preference for PROP was substantially influenced by the marked improvement in gray matter-white matter differentiation with PROP compared with SS-FSE imaging. Observer 1 agreed with this observation, though this observer believed that this preference did not strongly affect the detection of pathology in our specific patient group, as most patients were undergoing imaging for an evaluation of hydrocephalus.
The major advantage of PROP over SS-FSE imaging is an improvement in gray matter-white matter differentiation. In large part, this difference is thought to reflect differences in the length of the echo train. For PROP, most k-space is filled with early echoes, whereas for SS-FSE, the echoes tend to be much later and the result of a long echo train. Thus, in SS-FSE imaging, the data are very T2-weighted, and contrast between gray matter and white matter is poor.
Conclusion
PROP offers a feasible technique for imaging the unsedated pediatric brain. Its reduction of motion artifact is equal to that of SS-FSE imaging, but it also offers substantial improvement in parenchymal contrast in this specific patient population.
References
- 1.Ba-Ssalamaha A, Schick S, Heimberger K, et al. Ultrafast magnetic resonance imaging of the brain.Magn Reson Imaging 2000;18:237–243 [DOI] [PubMed] [Google Scholar]
- 2.Mittal TK, Halpin SF, Bourne MW, et al. A prospective comparison of brain contrast characteristics and lesion detection using single-shot fast spin-echo and fast spin-echo.Neuroradiology 1999;41:480–486 [DOI] [PubMed] [Google Scholar]
- 3.Sugahara T, Korogi Y, Hirai T, et al. Comparison of HASTE and segmented-HASTE sequences with a T2-weighted fast spin-echo sequence in the screening evaluation of the brain.AJR Am J Roentgenol 1997;169:1401–1410 [DOI] [PubMed] [Google Scholar]
- 4.Pipe JG. Motion correction with PROPELLER MR IMAGING: application to head motion and free-breathing cardiac imaging.Magn Reson Med 1999;42:963–969 [DOI] [PubMed] [Google Scholar]
- 5.Forbes KP, Pipe JG, Bird CR, et al. PROPELLER MR IMAGING: clinical testing of a novel technique for quantification and compensation of head motion.J Magn Reson Imaging 2001;14:215–222 [DOI] [PubMed] [Google Scholar]
- 6.Pipe JG. Improved in-plane correction for propeller MRI (abstract). In:Proceedings of the Ninth Annual Meeting of ISMRM, Glasgow, Scotland,2001:9
- 7.Schultz CL, Alfidi RJ, Nelson AD, et al. The effect of motion on two-dimensional Fourier transformation magnetic resonance images.Radiology 1984;152:117–121 [DOI] [PubMed] [Google Scholar]
- 8.Ehman RL, McNamara MT, Brasch RC, et al. Influence of physiologic motion on the appearance of tissue in MR images.Radiology 1986;159:777–782 [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Pediatrics Committee on Drugs. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures.Pediatrics 1992;89:1110–1115 [PubMed] [Google Scholar]
- 10.Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI.Magn Reson Med 1992;28:275–289 [DOI] [PubMed] [Google Scholar]
- 11.Meyer CH, Hu BS, Nishimura DG, et al. Fast spiral coronary artery imaging.Magn Reson Med 1992;28:202–213 [DOI] [PubMed] [Google Scholar]
- 12.Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures.Radiology 1989;173:255–263 [DOI] [PubMed] [Google Scholar]