Abstract
Summary: Meningocele is recognized as a rare, usually asymptomatic condition not associated with acute neurologic symptoms. We herein describe the case of a patient with a longstanding history of a lower back “mass” and recurrent syncope who became acutely unresponsive and developed bilateral retinal hemorrhages when she was placed in the supine position to undergo carotid sonography. MR imaging revealed a large, dorsal lumbar meningocele. The episode likely was caused by acutely increased intracranial pressure caused by displacement of CSF from the meningocele intracranially.
A simple dorsal meningocele is a protrusion of CSF and meninges into the subcutaneous tissue through a spinal defect. The overlying skin is usually intact (1). A complex meningocele represents a meningocele that is associated with other significant spinal anomalies (2). To the best of our knowledge, syncope and retinal hemorrhage are not reported manifestations of dorsal meningoceles.
Case Report
A 78-year-old right-handed woman had fallen onto her lower back without head injury or loss of consciousness at age 20 years. Shortly thereafter, she noticed a soft mass in her lower back that continued to grow until the age of 50 years. Six months after the fall, she experienced her first syncopal episode. She soon discovered that applying pressure to the mass caused a pressure sensation and ringing in her ears, blurred vision, and made her “see stars.”
Since then, she had experienced syncopal episodes approximately every 2 years, and each episode had been associated with inadvertent compression of the meningocele. She experiences transient headaches without any additional complaints after each of these syncopal episodes.
The patient had no symptoms of lumbosacral radiculopathy or lower back pain. These episodes were not associated with seizure activity or incontinence. The symptoms were present as long as the patient was in the supine or sitting position and disappeared without permanent deficit after she assumed a lateral decubitus or standing position.
The patient had been avoiding any pressure against the affected area, sleeping on her side, and using a special pillow when driving or sitting. The symptoms had been similar in severity until the episode described in this case report. The patient had undergone neurosurgical evaluation in 1981, and surgery was not recommended.
In January of 2002, the patient underwent carotid duplex sonography. The sonography technologist, despite the patient’s admonition regarding her condition, placed the patient in the supine position for the examination. The patient immediately lost consciousness and was unresponsive for several minutes. She regained consciousness after her husband lifted her upright. Immediately after the unresponsive episode, she was confused, had a headache, and lost vision in her left eye. No incontinence, weakness, or numbness occurred. Blood pressure and heart rate were not assessed.
Neurologic and ophthalmologic examinations revealed visual acuity of 2/200 in the left eye and intraretinal hematoma bilaterally, more pronounced on the left. After 1 month, her vision improved to 20/40 in the left eye with no residual retinal hemorrhage in either eye.
A physical examination revealed a large, easily compressible, nontender midsacral mass. The remaining results of the neurologic examination were normal.
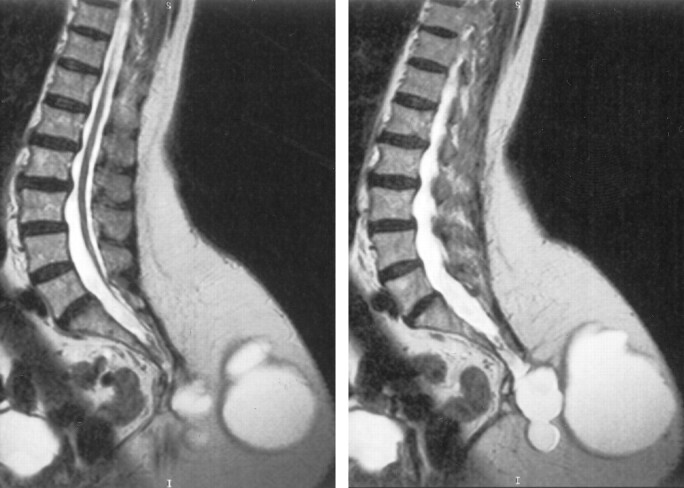
MR imaging of the lumbar spine was performed to further evaluate the mass. Axial and sagittal view T1- and T2-weighted images were obtained with the patient in the lateral decubitus position. The examination revealed a very large, lobulated meningocele, the largest component of which measured 10.7 × 10.2 × 11.0 cm (Fig 1). The meningocele originates from the caudal aspect of the sacrum and projects into the left side of the buttock. A tethered cord was present. Findings of MR imaging of the brain and carotid duplex sonography were unremarkable.
Fig 1.
Parasagittal view T2-weighted MR images show the large dorsal meningocele and the tethered cord.
Laboratory studies, including electrolytes, white blood cell count, creatine kinase, and troponin, were normal. Serum glucose was 198. EKG results were normal. These laboratory studies were ordered by the emergency department physician as part of a workup for syncope. Subsequently, the patient underwent a second neurosurgical evaluation and elected to defer surgery.
Discussion
Meningocele is a rare, usually asymptomatic, spinal anomaly. In this case, compression of the meningocele produced acute onset of clinical findings. We suggest that these findings are due to acutely increased intracranial pressure from displacement of extra-spinal CSF into the intracranial cavity.
The total average intracranial volume is 1700 mL. Brain, blood, and CSF are the components that occupy the intracranial cavity. Brain tissue makes up approximately 1200 mL of the total intracranial volume, CSF from 70 to 160 mL, and blood approximately 150 mL. The spinal subarachnoid CSF volume is 10 to 30 mL (3).
Weed proposed that the total intracranial volume of CSF, blood, and brain are relatively constant and changes in the volume of one are compensated by changes in others (4). Brain tissue is relatively noncompressible (5). Therefore, changes in CSF volume are compensated by changes in blood volume. When either volume exceeds the compensatory ability of the other, intracranial pressure will increase.
Normally, cerebral blood flow is constant with a cerebral perfusion pressure between 50 and 150 mm Hg or a mean arterial blood pressure of 60 to 160 mm Hg. When intracranial pressure rises above 40 to 50 mm Hg, cerebral perfusion pressure and cerebral blood flow may be diminished to a level that can cause loss of consciousness (3).
In this case, extra-spinal CSF (approximate total meningocele volume was 1200 mL) was displaced into the intraspinal and intracranial subarachnoid space. Even if only a fraction of the CSF contained within the meningocele was displaced intracranially, it may have led to a several-fold increase in the intracranial pressure with associated arrest of cerebral blood flow.
This case has important teaching points. First, not all cases of increased intracranial pressure (and its attendant signs and symptoms) are intracranial. Most research on the effects of increased intracranial pressure has used a model involving an intracranial mass or CSF flow obstruction as a cause for the elevated intracranial pressure. These are the causes that most often come to mind for physicians evaluating patients with symptoms of increased intracranial pressure. The cause for the elevated intracranial pressure in this case was extracranial and nonobstructive.
Second, the supine position of the patient at onset of syncope is very unusual. If this history is available, the radiologist may suggest a search for an extracranial source for the symptoms if such a search has not yet been conducted.
The short period of increased intracranial pressure required for the loss of consciousness (instantaneous) and retinal hemorrhages (several minutes) is very unusual. It is likely due to the acuteness of the change and/or the markedly elevated level of the intracranial pressure. These signs and symptoms may represent clinical manifestations of dorsal meningocele previously under-recognized and should be kept in the differential when evaluating patients with atypical histories of syncope or “increased intracranial pressure.”
References
- 1.Barkovich AJ. Congenital anomalies of the spine. In: Pediatric Neuroimaging. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;2000. :227–271
- 2.Naidich TP. The pediatric spine and cord: developmental and congenital abnormalities. In: Categorical Course on Spine and Cord Imaging. Oak Brook: American Society of Neuroradiology;1988. :1–15
- 3.Florence G, Seylaz J. Rapid autoregulation of cerebral blood flow: a laser-Doppler flowmetry study. J Cereb Blood Flood Metab 1992;12:674–680 [DOI] [PubMed] [Google Scholar]
- 4.Piper I. Intracranial pressure and elastance. In: Riley P, Bullock R, eds. Head Injury. 1997. :101–120
- 5.Brow JK. Mechanisms of production of raised intracranial pressure. In: Minns RA, ed. Problems of Intracranial Pressure in Childhood. London: MacKeith Press;1991. :13–35