Abstract
BACKGROUND AND PURPOSE: Cerebellar atrophy is considered the most prominent neuroradiologic finding in Marinesco-Sjögren syndrome (MSS). Our purpose was to investigate this neuroradiologic feature in a series of patients with MSS.
METHODS: Five patients with MSS (age range, 5–19 years) underwent native MR imaging of the brain. The findings were assessed with particular attention to the cerebellum and the supratentorial structures.
RESULTS: Only two patients had slight cerebellar atrophy; the cerebellum was normal in size and configuration in the other patients. Additional supratentorial findings were present in some of the patients, with an apparently small anterior pituitary gland in two and the absence of the posterior pituitary bright spot in three of the patients.
CONCLUSION: Cerebellar atrophy is not an obligatory finding in MSS, and almost normal cranial MR imaging results are compatible with the diagnosis. Morphologic changes of the pituitary gland seem to be common in patients with MSS and are not associated with endocrine dysfunction.
Marinesco-Sjögren syndrome (MSS) (MIM 248800) is a rare autosomal recessive disorder characterized by bilateral cataracts, cerebellar ataxia, developmental delay, and nonprogressive mental retardation (1, 2). Additional features include a short stature; hypogonadism; skeletal deformities; and variable neuromuscular manifestations, such as myopathy (3–5) and motor and sensory neuropathy (6, 7). Myopathy may be chronic, or it may occur as acute parainfectious rhabdomyolysis (8). The disorder occurs in patients of diverse ethnic backgrounds. MSS shows a genetic identity with the congenital cataract facial dysmorphism neuropathy (CCFDN) syndrome (9). CCFDN (MIM 604168) is an autosomal recessive disorder primarily found in Bulgarian Roma families (10). The MSS/CCFDN gene maps to chromosome region 18q23 (11). The gene of this multisystemic disorder is still unknown, and the diagnosis is based on family history and clinical and neuroradiologic findings. There are no diagnostic laboratory tests for MSS. A low concentration of vitamin E due to chylomicron retention disease has been reported in two Italian boys with MSS (12). The most striking neuroradiologic feature described in the literature is a severe cerebellar atrophy, especially of the vermis, and a small posterior fossa (13, 14). Other supratentorial abnormalities may be present such as cortical atrophy, white matter lesions, a small anterior pituitary gland, and nonvisualization of the posterior pituitary gland (14, 15).
MR imaging findings are considered important for the diagnosis of MSS. In particular, cerebellar atrophy seems to be a consistent finding, with only few described exceptions. However, the number of studied cases is small, and the evaluation of a larger series of patients is recommended to confirm the neuroradiologic features of MSS (14).
Cerebellar atrophy is considered the most prominent neuroradiologic finding in MSS. Our purpose was to investigate this neuroradiologic feature in a series of patients with MSS.
Methods
Patients
Between 1986 and 2001, we diagnosed MSS in 14 patients. All patients were from Roma families, originating from the same area in Serbia north of Belgrade. The prevalence of consanguinity among these families is high. The disease shows an autosomal recessive mode of inheritance. We demonstrated an identical, conserved haplotype in the chromosomal region of the MSS gene in all 14 patients.
Five of these patients agreed to undergo a detailed evaluation of fat metabolism and endocrine function, as well as cranial imaging studies. The identical conserved haplotype was proved in each of them. The patients were all male between 5 and 19 years of age, and among them were two brothers 12 and 19 years olds. All patients had an unremarkable peri- and postnatal history. They all had had bilateral cataracts that had been surgically removed in their first months of life. Motor development was delayed in all patients. They are all mentally impaired, but mental deficits remained only mild and nonprogressive. None of the patients had seizures. They had no episodes of parainfectious myalgia or myoglobinuria. All patients had clear signs of a central movement disorder with a wide-based gait, dysarthria, truncal ataxia, and extrapyramidal signs (eg, chorea). Mild muscular hypotonia was present, no clonus was present, and deep tendon reflexes were diminished or absent. Nerve conduction velocities were delayed in all patients, indicating a demyelinating neuropathy. One patient was wheelchair bound.
Hypogonadism had been ruled out in the pubertal patients. In the 19-year-old patient, luteinizing hormone and follicle-stimulating hormone levels were low for his age, but his genital development was normal and radiographs of the left hand and wrist showed an adequate bone age. Insulinlike growth factor-1 level was within the normal to low normal range in all patients. Fat malabsorption and deficiency of the fat soluble vitamins were ruled out in all five patients. Laboratory values for T3, T4, thyroid-stimulating hormone and cortisol, as well as for serum electrolytes, creatinine, transaminases, bilirubin, and serum creatine kinase, were normal in all patients. Peripheral blood karyotypes and findings from urine metabolic screening were normal.
Imaging
Cranial MR imaging examinations were performed by using a 1.5-T MR unit (Signa; GE Medical Systems, Milwaukee, WI). Nonenhanced transaxial T1- (400/16[TR/TE]), T2- (3200/256), and proton density-weighted (3440/15.2)images; sagittal T1- or T2-weighted images; and coronal fluid-attenuated inversion recovery (FLAIR) (9002/142) images were obtained. The section thickness was generally 5 mm. In addition, 3-mm sagittal T1-weighted images were obtained in a case in which the bright spot in the posterior pituitary gland was not visualized. We had obtained informed consent for an investigation without anesthesia; therefore, the complete sequence of images could not be obtained in some of the patients. The cerebellum was evaluated for the size of the cerebellar hemispheres and vermis, for enlargement of the fourth ventricle and cisterna magna, for the size of the posterior fossa, for preservation of normal foliation, and for widening of the cerebellar fissures. Cortical atrophy was indicated by widening of external CSF space, and leukoencephalopathy showed increased signal intensity in the white matter on T2-weighted images. Two authors (I.S., R.L.) independently evaluated the images.
Results
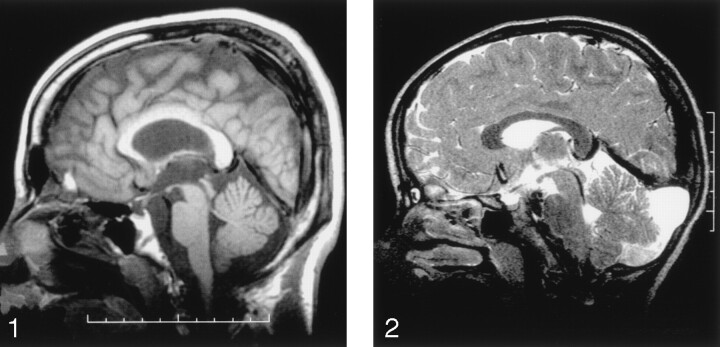
MR findings are listed in Table 1, and selected images appear in Figures 1 and 2. Patients 4 and 5 had slight cerebellar atrophy with prominent fissures in the superior part of the vermis (Fig 1). The other patients had no cerebellar atrophy, and the vermis and the cerebellar hemispheres appeared normal in size and configuration on axial and sagittal sections. Patients 1 and 2 had an enlarged cerebellomedullary cisterna (Fig 2) without cerebellar atrophy. Patients 4 and 5 had leukoencephalopathy with periventricular and juxtacortical focal lesions that were hyperintense on the T2-weighted and FLAIR images. In addition, patient 5 had diffuse cortical-subcortical atrophy. The anterior pituitary gland was small in patients 4 and 5. The hyperintensity of the posterior pituitary gland on the T1-weighted image could not be visualized in patients 2, 4, and 5 (Fig 1). The posterior pituitary gland could not be assessed in the other two patients, because sagittal T1-weighted sections were not acquired.
TABLE 1:
MRI findings in five patients with MSS
| Patient No./Age | Cerebellar Atrophy | Enlarged Cerebellomedullary Cisterna | Cortical Atrophy | Leukencephalopathy | Hypoplastic Anterior Pituitary Gland | Hyperintense Posterior Pituitary Gland |
|---|---|---|---|---|---|---|
| 1/5 y | No | Yes | No | No | No | No data |
| 2/6 y | No | Yes | No | No | No | No |
| 3/9 y | No | No | Possibly yes | No | No | No data |
| 4/12 y | Possibly, vermis | No | No | Unilateral, 2 lesions, juxtacortical | Yes | No |
| 5/19 y | Possibly, vermis | No | Yes | Bilateral lesions, periventricular and juxtacortical | Yes | No |
Fig 1.
Sagittal midline T1-weighted MR image shows slight cerebellar atrophy of the superior part of the vermis and no demonstrable posterior pituitary bright spot. The anterior pituitary gland appears small.
Fig 2.
Sagittal midline T2-weighted MR image shows that the cerebellomedullary cisterna is enlarged and reaches up to the tentorium. No cerebellar atrophy is observed.
Discussion
Our patients presented with the characteristic clinical features of the MSS. We demonstrated an identical haplotype in the chromosomal region of the MSS gene (9) in all patients. The diagnosis of MSS is based primarily on the clinical picture and is supported by the family history and neuroimaging results. Distinct cerebellar atrophy, especially of the vermis, is considered the most striking neuroradiologic finding in MSS. The literature describes this as a relatively constant finding, but the number of cases in most published reports is small (Table 2). Often these are only case reports. All patients with MSS have striking cerebellar atrophy, as shown on MR images (3, 12, 17, 23). McLaughlin et al (14) and Georgy et al (13) reported on neuroradiologic findings in patients with MSS; the number of cases in their series were three and eight, respectively. All their patients had a small cerebellar vermis, and nine of the patients also had small cerebellar hemispheres. Georgy et al (13) demonstrated, in four patients, that the cerebellar atrophy was nonprogressive over a span of 7 years. These groups both concluded that MR imaging may be helpful in the early diagnosis of the disorder. Only two of our patients had slight cerebellar atrophy affecting the vermis. Our investigation and single reports in the literature (Table 2) show that the cerebellar atrophy is not a definitive finding in MSS.
TABLE 2:
Neuroradiologic findings and presence of neuropathy and/or myopathy in MSS patients reported in the literature
| Reference and No. of Patients | Cerebellar Atrophy | Cortical Atrophy | Leukencephalopathy | Pituitary Gland Changes | Neuropathy | Myopathy |
|---|---|---|---|---|---|---|
| Sasaki et al (3), n = 1 | 1 | 0 | 0 | Unknown | 0 | 1 |
| Farah et al (16), n = 2 | 2 | 2 | 0 | Unknown | 2 | 2 |
| Ishikawa et al (17), n = 1 | 1 | 0 | 0 | Unknown | 0 | 1 |
| Bromberg et al (15), n = 2 | 1 | 2 | 2 | Unknown | 2, mild | 2, mild |
| Superneau et al (4), n = 17 | 17 | 0 | 0 | Unknown | 0 | 17 |
| McLaughlin et al (14), n = 3 | 3 | 1 | 1 | 3 | 0 | 0 |
| Georgy et al (13), n = 8 | 8 | 0 | 0 | Unknown | 0 | 8 |
| Müller-Felber et al (8), n = 4 | 2, slight | 0 | 0 | Unknown | 4 | 4, acute |
| Aguglia et al (12), n = 2 | 2 | 0 | 0 | Unknown | 2 | 0 |
| Hakamada et al (6), n = 1 | 1, slight | 0 | 0 | Unknown | 1 | 0 |
| Williams et al (18), n = 1 | Unilateral dysplasia | 1 | 0 | Unknown | 0 | 0 |
| Komiyama et al (19), n = 4 | 4 | 0 | 0 | Unknown | 4 | 0 |
| Katafuchi et al (20), n = 1 | 1 | 1 | 0 | Unknown | 0 | 1/1 |
| Walker et al (21), n = 4 | 0 | 1 | 0 | Unknown | 0 | 4 |
| Dotti et al (22), n = 3 | 3 | 0 | 0 | Unknown | 0 | 3 |
The posterior pituitary gland is normally visualized on T1-weighted images as a circumscribed hyperintense echogenic area (24). In three of the five patients with MSS, the hyperintensity of the posterior pituitary gland could not be visualized. Nonvisualization of the posterior pituitary gland was previously described in three patients with MSS (14), and it seems to be common in MSS. In those cases, the posterior pituitary gland is usually ectopically located at the base of the hypothalamus (25) We could not detect ectopia of the posterior bright spot on the 3-mm sagittal sections. The appearance of the posterior pituitary bright spot is also considered to be dependent on the patient’s hydration, and it might be absent in cases of severe dehydration. Dehydration was not present in the five patients, according to clinical and laboratory findings. In the two patients with a hypoplastic anterior pituitary gland, we excluded endocrine dysfunction.
Cortical atrophy (in two of our five patients) and periventricular white matter lesions (in two of the five patients) have also been previously described in MSS (10, 14). In agreement with the literature (14), these two findings were most pronounced in the oldest patient (patient 5). Because of the lack of follow-up studies, whether the changes are progressive or whether the distribution is only coincidental remains unclear.
Recently, investigators have demonstrated that MSS with myoglobinuria and CCFDN both map to 18q23 (9). The patients with MSS in that study resembled ours in terms of the clinical aspects, with the exception of myoglobinuria and neuroradiologic findings. The CCFDN phenotype overlaps with that of MSS (11). Neuropathy is one of the cardinal features of CCFDN and only occasional in MSS. On the other hand, cerebellar involvement is only mild and not mandatory for the diagnosis of CCFDN (10). Myopathy may be present in patients with MSS (3, 4, 8) but not in patients with CCFDN. These differences originally justified their classification as two distinct disease entities. Neuroradiologic findings in patients with CCFDN are cerebral and spinal cord atrophy and occasional focal lesions (11). That no patients with severe cerebellar atrophy were involved in the MSS/CCFDN study must be considered.
Conclusion
MSS is a rare autosomal recessive disorder involving congenital cataracts, cerebellar ataxia, developmental delay, and variable neuromuscular findings. Severe cerebellar atrophy is not an obligatory neuroradiologic finding, as many previous authors have suggested.
Analysis of the MSS cases published to date and of the genetic identity of CCFDN and MSS with myoglobinuria suggest the existence of one MSS phenotype with myopathy and severe cerebellar atrophy and a second neuropathic phenotype with only slight changes in the posterior fossa. Further molecular genetic studies are necessary to determine whether the variability of neuroradiologic findings in MSS is related to phenotype or genotype variability.
Footnotes
Supported by the Deutsche Forschungsgemeinschaft, project BU 1291/1.
This subject was presented as a poster at the Jahrestagung der Deutschen Gesellschaft für Neuropädiatrie in Freiburg, Germany, April 6, 2002.
References
- 1.Marinesco G, Draganesco S, Vasiliu D. Nouvelle maladie familiale caracterisée par une cataracte congénitale et un arret du développement somato-neuro-psychique. Encephale 1931;26:97–109 [Google Scholar]
- 2.Sjögren T. Hereditary congenital spinocerebellar ataxia accompanied by congenital cataract and oligophrenia. Confinia Neurol 1950;10:293–308 [PubMed] [Google Scholar]
- 3.Sasaki K, Suga K, Tsugawa S, et al. Muscle pathology in Marinesco-Sjögren syndrome: a unique ultrastructural feature. Brain Dev 1996;18:64–67 [DOI] [PubMed] [Google Scholar]
- 4.Superneau D, Wertelecki W, Zellweger H, Bastian F. Myopathy in Marinesco-Sjogren Syndrome. Eur Neurol 1987;26:8–16 [DOI] [PubMed] [Google Scholar]
- 5.Torbergsen T, Stalberg E, Aasly J, Lindal S. Myopathy in Marinesco-Sjogren syndrome: an electrophysiological study. Acta Neurol Scand 1991;84:132–138 [DOI] [PubMed] [Google Scholar]
- 6.Hakamada S, Sobue G, Watanabe K, Kumagai T, Hara K, Miyazaki S. Peripheral neuropathy in Marinesco-Sjogren syndrome. Brain Dev 1981;3:403–406 [DOI] [PubMed] [Google Scholar]
- 7.Zimmer C, Gosztonyi G, Cervos-Navarro J, von Moers A, Schröder J. Neuropathy with lysosomal changes in Marinesco-Sjögren syndrome: fine structural findings in skeletal muscle and conjunctiva. Neuropediatrics 1992;23:329–335 [DOI] [PubMed] [Google Scholar]
- 8.Müller-Felber W, Zafiriou D, Scheck R, et al. Marinesco Sjögren syndrome with rhabdomyolysis: a new subtype of the disease. Neuropediatrics 1998;29:97–101 [DOI] [PubMed] [Google Scholar]
- 9.Merlini L, Gooding R, Lochmüller H, et al. Genetic identity of Marinesco-Sjögren/myoglobinuria and CCFDN syndromes. Neurology 2002;58:231–236 [DOI] [PubMed] [Google Scholar]
- 10.Tournev I, Kalaydjieva L, Youl B, al. e. Congenital cataracts facial dysmorphism neuropathy syndrome, a novel complex genetic disease in Balkan gypsies: clinical and electrophysiological observations. Ann Neurol 1999;45:742–750 [PubMed] [Google Scholar]
- 11.Angelicheva D, Tournev I, Dye D, Chandler D, Thomas P, Kalaydjieva L. Congenital cataracts facial dysmorphism neuropathy (CCFDN) syndrome: a novel developmental disorder in Gypsies maps to 18qter. Eur J Hum Genet 1999;7:560–566 [DOI] [PubMed] [Google Scholar]
- 12.Aguglia U, Annesi G, Pasquinelli G, et al. Vitamin E deficiency due to chylomicron retention disease in Marinesco-Sjögren syndrome. Ann Neurol 2000;47:260–264 [PubMed] [Google Scholar]
- 13.Georgy B, Snow R, Brogdon B, Wertelecki W. Neuroradiologic findings in Marinesco-Sjögren syndrome. AJNR Am J Neuroradiol 1998;19:281–283 [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin J, Pagon R, Weinberger E, Haas J. Marinesco-Sjögren syndrome: Clinical and magnetic resonance imaging features in three children. Dev Med Child Neurol 1996;38:636–644 [DOI] [PubMed] [Google Scholar]
- 15.Bromberg M, Junck L, Gebarski S, McLean M, Gilman S. The Marinesco-Sjogren syndrome examined by computed tomography, magnetic resonance, and 18F-2-fluoro-2-deoxy-D-glucose and positron emission tomography. Arch Neurol 1990;47:1239–1242 [DOI] [PubMed] [Google Scholar]
- 16.Farah S, Sabry M, Khuraibet A, et al. Marinesco-Sjögren syndrome in a Bedouin family. Acta Neurol Scand 1997;96:387–391 [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa T, Kitoh H, Awaya A, Nonaka I. Rapid cataract formation in Marinesco-Sjögren syndrome. Pedriatr Neurol 1993;9:407–408 [DOI] [PubMed] [Google Scholar]
- 18.Williams T, Buchhalter J, Sussman M. Cerebellar dysplasia and unilateral cataract in Marinesco-Sjögren syndrome. Pediatr Neurol 1996;14:158–161 [DOI] [PubMed] [Google Scholar]
- 19.Komiyama A, Nonaka I, Hirayama K. Muscle pathology in Marinesco-Sjogren syndrome. J Neurol Sci 1989;89:103–113 [DOI] [PubMed] [Google Scholar]
- 20.Katafuchi Y, Kosai K, Ohtaki E, et al. Cerebral cortex and brainstem involvement in Marinesco-Sjogren syndrome. Ann Neurol 1990;27:448–449 [DOI] [PubMed] [Google Scholar]
- 21.Walker P, Blitzer M, Shapira E. Marinesco-Sjögren syndrome: evidence for a lysosomal storage disorder. Neurology 1985;35:415–419 [DOI] [PubMed] [Google Scholar]
- 22.Dotti M, Bardelli A, De Stefano N, et al. Optic atrophy in Marinesco-Sjogren syndrome: an additional ocular feature—report of three cases in two families. Ophtalmic Pediatr Genet 1993;14:5–7 [DOI] [PubMed] [Google Scholar]
- 23.Shimizu T, Matsuishi T, Yamashita Y, Koga Y. Mariensco-Sjogren syndrome: can the diagnosis be made prior to cataract formation? Muscle Nerve 1997;20:909–910 [PubMed] [Google Scholar]
- 24.Ultmann M, Siegel S, Hirsch W, Finegold D, Foley T. Pituitary stalk and ectopic hyperintens T1 signal on magnetic resonance imaging: implications for anterior pituitary dysfunction. Am J Disease Child 1993;147:647–652 [DOI] [PubMed] [Google Scholar]
- 25.Abrahams J, Trefelner E, Boulware S. Idiopathic growth hormone deficiency: MR findings in 35 patients. AJNR Am J Neuroradiol 1991;12:155–160 [PMC free article] [PubMed] [Google Scholar]